- Types of malignancy
- Reasons for development
- Manifestations and symptoms
- Diagnostics
- Treatment options
- Prognosis factors
GI stromal tumors (GISTs) are neoplasms with varying malignant potential, from essentially indolent (low-grade) tumors to rapidly progressive malignancies.
GISTs occur throughout the intestinal tract, and most contain an activating mutation in KIT or platelet-derived growth factor A (PDGFRA). Diagnosis is made by immunohistochemistry, but molecular analysis with mutational analysis is of paramount importance to select appropriate therapy. Approximately 50-70% of GISTs originate from the stomach. The small intestine becomes the second most common site, with 20-30% of GISTs. Less common sites of occurrence include the colon, rectum (5-15%), and esophagus (<5%).
Distant metastases appear late in most cases. Unlike other soft tissue tumors, the common metastatic sites of GIST are the liver and peritoneum. The average age at diagnosis is 50 to 60 years.
Classification
Classification based on histological assessment of the cellular composition of the tumor characteristic of unclassified soft tissue sarcomas (epithelioid, spindle cell, etc.) is now irrelevant.
Instead of histological evaluation of GIST, the mitotic index is used.
Gastrointestinal stromal tumors are usually divided according to the level of aggressiveness into three subtypes:
- benign/indolent;
- uncertain malignant potential;
- malignant.
The assignment of a specific tumor to one or another subtype is done through a comprehensive assessment of four indicators.
- Tumor size. Large tumors are more likely to behave aggressively than small tumors. A small GIST measuring less than 2 cm may not cause problems in the future.
- Mitotic index. The mitotic count is the number of actively dividing cells visible under a microscope in a given area of the tumor. It tells doctors about the rate at which cancer cells are multiplying and serves as an indicator of the aggressiveness of the tumor. An index of ≤ 5/50 is considered low, which corresponds to 5 visible divisions in a field of 5 mm².
- Location. Tumors in the small intestine and rectum are more aggressive than tumors in the stomach.
- Tumor rupture. If the surface of the tumor was damaged during surgery to remove it, or if the tumor was not intact at the time of surgery, there is a possibility that tumor cells may have invaded the abdominal cavity. Sometimes this alone identifies the tumor as high risk.
What are gastrointestinal stromal tumors
Gastrointestinal stromal tumor (GIST) is a mesenchymal formation of a malignant nature.
There is an assumption that it develops from Cajal cells or their predecessors. This tumor is similar to smooth muscle and neurogenic formations. However, upon deeper examination, certain features are revealed that make it possible to classify these neoplasms as a separate group.
Important! All types of GIST are potentially malignant and metastasize primarily through the hematogenous route.
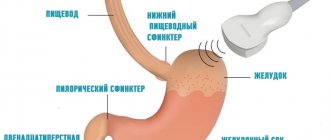
GIST is a node located under the mucosa. Such formations tend to grow into the lumen of a hollow organ. How dangerous a tumor is depends on its location, size and rate of division.
In GIST, the size of which does not exceed 2-5 cm, the malignant potential is quite low, and in formations with a diameter of more than 10 cm it is noticeably higher.
Each clinical case has its own GIST treatment tactics. A mandatory aspect of treatment is the search for mutations in genes. When they are identified, targeted therapy is used using low-molecular tyrosine kinase inhibitors and a multikinase inhibitor. This treatment is carried out in several stages.
Causes and risk factors
Scientists have discovered that cells can grow uncontrollably as a result of a disruption in their DNA. In GIST, a specific mutation causes a cellular enzyme known as KIT to be constantly turned on.
KIT is an enzyme (from the group of intracellular tyrosine kinases) responsible for transmitting growth and survival signals within the cell. If it is turned on, the cell grows or multiplies. Turning off this enzyme puts the cell into a state of rest. The hyperactive, uncontrolled mutant enzyme KIT triggers the rapid growth and endless proliferation of GIST tumor cells.
Understanding this process has already helped identify new treatments for this neoplasia.
Risk factors for GIST
Neurofibromatosis
The most significant risk factor for GIST is the presence of neurofibromatosis. It is almost always associated with the development of such neoplasms.
Family history
There are rare cases of hereditary GISTs. Hereditary tumors are characterized by inherited germline mutations in KIT or PDGFRA and additional syndromes such as:
- skin hyperpigmentation;
- irritable bowel syndrome;
- dysphagia;
- intestinal diverticulosis.
95% of GISTs are KIT positive. But 5% show no detectable KIT expression. In a subset of these KIT-negative GISTs, mutations occur in the PDGFRA gene rather than in KIT. PDGFRA immunostaining has been shown to help differentiate between KIT-negative GISTs and other gastrointestinal mesenchymal lesions.
BRAF and protein kinase Cθ (PKCtheta) mutations have also been reported in a small number of non-KIT/PDGFRA GISTs. Initial studies indicate that BRAF-mutant GISTs have a predilection for the small bowel and are not associated with a high risk of malignancy.
Causes of stromal tumors
In most cases, gastrointestinal stromal tumors appear spontaneously. Up to 85% of GISTs have mutations in the C-KIT gene and 3-18% in the PDGFRA gene. In 10-15% of cases, mutations in these genes are not detected, so the pathology is assigned wild type (WT).
But broader genetic studies suggest that WT stromal tumors are a heterogeneous group that harbor activating mutations in a number of genes.
Reference! Much less frequently, GIST occurs as part of hereditary familial or idiopathic multitumor syndromes (Carney's triad).
Symptoms
Up to 75% of GISTs are detected when they are less than 4 cm in diameter and are either asymptomatic or associated with nonspecific symptoms. They are often diagnosed incidentally during X-ray examinations or endoscopic or surgical procedures performed to investigate gastrointestinal disease or to treat emergency conditions. In Japan, mass screening for gastric adenocarcinoma using upper endoscopy has led to an increase in incidental detection of asymptomatic GISTs.
In a population-based study, the average size of GIST tumors that were detected as incidental findings was 2.7 cm, compared with 8.9 cm for those that were detected based on symptoms.
Upper gastrointestinal bleeding is the most common clinical manifestation of GIST. It is observed in 40-65% of patients. Patients who have experienced significant blood loss may report malaise, fatigue, or shortness of breath on exertion.
Obstruction may result from intraluminal growth of an endophytic tumor or from luminal compression due to an exophytic lesion. Obstructive symptoms may be organ-specific (for example, difficulty swallowing with an esophageal tumor, constipation with a rectal tumor, obstructive jaundice with a duodenal tumor).
Other symptoms:
- abdominal pain;
- anorexia;
- nausea;
- vomit;
- weight loss;
- epigastric pressure;
- fast saturation.
Diagnostics
The diagnosis is made taking into account clinical symptoms, external examination data and objective studies. Patients are prescribed radiography of the abdominal organs, ultrasound and contrast CT of the abdominal organs. For low-lying tumors, patients are referred for MRI. If the stomach is affected, gastroscopy is performed, if the large intestine is affected, colonoscopy is performed. If metastasis of a gastrointestinal stromal tumor is suspected, chest radiography, chest CT, spinal radiography, skeletal bone scintigraphy and other studies are performed. Whenever possible, PET-CT is used, which makes it possible to accurately determine the extent of gastrointestinal stromal tumors and identify small metastases that are not detected using other techniques. The final diagnosis is established on the basis of histological and immunochemical examination of a tissue sample taken during an endoscopic examination.
Treatment
Targeted therapy
Although surgical resection is the preferred treatment for gastrointestinal stromal tumors (GISTs) and offers the only chance for complete cure, tyrosine kinase inhibitors (TKIs) now play a major role in treatment. And the most common drugs in this group are Gleevec® and Sutent®. Imatinib and sunitinib, respectively.
Now, when the tumor is resistant to treatment with these drugs, new therapeutic agents such as regorafenib are widely used, and sorafenib and others are widely used in the United States and Europe.
Standard indications for the use of tyrosine kinase inhibitors:
- treatment of inoperable and/or metastatic GISTs;
- preoperative treatment of tumors that may become resectable with negative margins;
- adjuvant treatment after complete gross resection of neoplasia to prevent recurrence.
The European Society of Medical Oncology (ESMO) recommends that assessment of response to treatment is complex and that early progression in particular should be confirmed by an experienced team. In most cases, antitumor activity is indicated by tumor shrinkage. But sometimes the tumor response is determined only by a decrease in its density. Or a decrease in density (with an increase in size!) may precede subsequent tumor shrinkage.
According to ESMO, lack of tumor progression after 6 months of treatment is also a tumor response.
Surgery
Until recently, the only treatment for GIST was surgery. The goal of the operation is to completely remove the tumor. However, surgery alone for large or spread tumors gives disappointing results.
Surgery has remained the definitive therapy for well-localized GISTs. Despite the proven success of imatinib and other newer tyrosine kinase inhibitors, surgical resection remains the first choice and offers the only chance for complete clearance of GISTs.
Given the ability of imatinib to transform inoperable tumors into partially resectable ones, cytoreductive surgery can be used - removing the maximum possible volume of the tumor in order to achieve incomplete remission. The patient receives neoadjuvant therapy for 6 months and then undergoes wide resection. This often provides a significant increase in survival.
Treatment of gastrointestinal stromal tumors
With a clear, non-scattered localization of gastrointestinal stromal tumors, treatment begins with surgery. In case of unresectable formations, resumption of the disease after remission, or in the presence of metastases, targeted therapy is preferred.
Localized forms
For small tumors (less than 1-2 cm), if the clinical situation allows, monitoring the patient over time is justified.
However, having abandoned active surgical tactics, it is necessary to take into account the potential risk of pathology progression, based on histological studies. If the formation is more than 2 cm, then radical intervention is indicated.
The nuances of therapy using surgical methods:
- If the tumor is located in the esophagus, inlet of the stomach, duodenum or rectum, therapy is recommended before resection to reduce its size. And then an operation is performed to preserve the organ.
- If there are doubts about the operability of the tumor, then before the planned radical intervention it is recommended to take imatinib for 6-12 months.
- Patients with a low risk of pathology progression are monitored after surgery. If the risk is high or intermediate, then drug therapy with imatinib is used for 1-3 years.
After a full course of adjuvant therapy, in case of disease relapse, imatinib is resumed again.
Disseminated tumors and other cases
In disseminated cases of GIST or in the absence of the possibility of resection, 3 lines of targeted therapy are sequentially carried out:
- Imatinib is a first-line drug. It is a low-molecular-weight inhibitor of a number of receptor (c-KIT, PDGFRα) and non-receptor tyrosine kinases (Abl, Bcr-Abl). The development of primary or secondary resistance to this substance is possible.
- Sunitinib is a second-line drug. It belongs to low-molecular inhibitors of various (more than 80) tyrosine kinases. However, due to severe toxicity, some patients interrupt treatment with this substance.
- Regorafenib is a third-line drug. It is an oral multitarget (multikinase) inhibitor acting on tyrosine kinases, angiogenesis and the tumor microenvironment. Regorafenib is prescribed to patients who were forced to stop taking sunitinib due to intolerance.
In the majority of scattered cases of GIST, resistance to imatinib and sunitinib develops, against which the disease begins to rapidly progress.
Regorafenib is the main solution to the problem if the first 2 drugs no longer work. Thanks to this, patients receive long-term, successful therapy.
Typically, treatment begins with the use of imatinib, gradually replacing the dosage of 400 mg to 800 mg per day. At the next stage, they move on to sunitinib and only then to regorafenib.
However, in case of increased sensitivity to sunitinib, regorafenib, which has less toxicity to the body, can be used already in the second line.
Benefits of treatment in Belgium
- Availability of the latest drugs. In Belgium, if the tumor is resistant to therapy with imatinib, sunitinib and regorafenib, the patient has the opportunity to receive treatment with new drugs - sorafenib, dasatinib and nilatanib. This helps prolong life even in the most difficult cases.
- Laparoscopic minimally invasive surgery. It is used in Belgium for tumors not exceeding 5 cm, located in the stomach and small intestine. Advantages of laparoscopic resection include faster resumption of normal nutrition, shorter hospital stay after surgery, and less use of analgesia. The short-term results remain the same as with open surgery.
- Cost of treatment. It is approximately 30% lower in Belgium than in neighboring European countries and 60% lower than in the United States. Taking into account the high cost of drugs, this fact is of particular importance.
Symptoms of gastrointestinal stromal tumors
GIST has no specific manifestations. In the early stages, most stromal tumors have no symptoms. Even fairly large formations of this type often do not make themselves felt.
But over time, as with other malignant tumors of the gastrointestinal tract, patients experience: abdominal pain, nausea, causeless weight loss, increased fatigue.
An ulcer may appear on the mucous membrane under which the tumor is located, causing hidden or massive bleeding in the gastrointestinal tract. This leads to the development of hematological syndrome, characterized by impaired hemoglobin synthesis due to iron deficiency.
A stomach tumor localized in the antrum can provoke a pathological narrowing of the outlet section of this organ.
Gastrointestinal stromal tumors (GIST) as an independent disease were described 20 years ago. GIST is the most common mesenchymal tumor of the gastrointestinal tract; its detection rate reaches 15 per 1 million population per year [1,2,21]. Most often, GIST occurs in the stomach, where up to 60-70% of these neoplasms are detected [4, 5, 10, 13, 14, 23]. GISTs develop from Cajal cells, which are pacemaker cells of the gastrointestinal tract [20]. Differential diagnosis with other nonepithelial tumors of the gastrointestinal tract is based on immunohistochemical examination. These tumors are characterized by positive reactions with markers CD117, CD34, smooth muscle actin, desmin and S100 [6].
The widespread clinical use of imatinib mesylate (Gleevec) has changed the tactics of treating patients and led to a revision of the indications for surgical treatment of GIST [8]. However, radical surgical removal of the primary tumor is still considered the most preferable treatment option [3,7].
From July 2005 to September 2010 at the Institute of Surgery named after. A.V. Vishnevsky examined and treated 34 patients with morphologically verified GISTs. Tumors were localized in the stomach in 17 (53.1%) of them, in the duodenum in 10 (31.3%) and in the small intestine in 5 (15.6%) patients. The size of primary neoplasms varied widely and averaged 6.7±5.4 cm.
We present an observation of the treatment of giant gastric GIST.
Patient K.,
70 years old, 07/06/10 entered the Department of Abdominal Surgery No. 1 of the Institute of Surgery named after. A.V. Vishnevsky with complaints of pain and a feeling of heaviness in the epigastric region, the presence of a tumor-like formation in the abdomen, weight loss of 6 kg in a year.
From the anamnesis it is known that the patient had been bothered by periodic pain in the epigastric region for a year. Six months before admission, the patient independently discovered a tumor-like formation in the abdominal cavity. A computed tomography scan performed on an outpatient basis revealed a tumor of the abdominal cavity of a heterogeneous structure, measuring 24.5×22.5×14.4 cm.
Upon admission, the patient’s condition was satisfactory, the skin was naturally colored and clean. Vesicular breathing in the lungs is carried out symmetrically, there is no wheezing. Heart sounds are sonorous and rhythmic. Blood pressure 130/75 mm Hg. Pulse 72 per minute. The abdomen is not swollen, participates in breathing in all parts, is deformed due to the presence of a tumor with a diameter of about 30 cm. On palpation, the tumor is rocky in density, painless, and moves slightly. Peristalsis is normal. Regular stool. No rectal pathological changes were detected. There are no dysuric phenomena.
There are no deviations from the norm in general blood and urine tests and biochemical blood tests.
Ultrasound in the abdominal cavity visualizes a round-shaped cystic formation with clear, uneven contours. On the inner surface, solid soft tissue structures of the type of outgrowths are determined. The wall of the formation appears layered, unevenly thickened to 13 mm. The formation displaces the intestinal loops posteriorly and the head of the pancreas downward. There is no pyelectasis.
According to 256-multidetector spiral computed tomography of the abdominal organs, no free or encysted fluid was detected. From the lower surface of the liver to the pelvic aperture, a tumor-like formation with clear contours, irregular oval shape, measuring 30x32x28 cm is visualized (Fig. 1, a).
Figure 1. Multidetector spiral computed tomography of the abdominal organs. a — arterial phase of the study. A tumor with clear contours, oval in shape, up to 32 cm in size. 1 - tumor, 2 - stomach, 3 - duodenum. The structure of the formation is heterogeneous: against the background of the soft tissue component, hypodensity areas are visualized. With contrast enhancement, a 3 mm thick capsule is visible, unevenly accumulating the contrast agent. Soft tissue structures also accumulate contrast agent. The formation is adjacent with its medial contour to the outlet of the stomach. The stomach is enlarged and distended with fluid. The fatty layer between the identified formation and the wall of the gastric outlet cannot be traced. The posterior surface of the formation is adjacent to the head and isthmus of the pancreas, pushing them downward; there are no signs of germination into the gland. The duodenum is spread out on the tumor. The gallbladder and loops of the small intestine are pushed aside, but there is no tumor growth in them. The left and right hepatic arteries pass along the contour of the formation and are not involved in the tumor. The common hepatic artery, splenic artery, right and left branches of the portal vein, and splenic vein are intact (Fig. 1, b).
Figure 1. Multidetector spiral computed tomography of the abdominal organs. b — the structure of the formation is heterogeneous: against the background of the soft tissue component, hypodensity areas are visualized. Thus, the detected formation corresponds to GIST of the stomach or retroperitoneal tumor.
Magnetic resonance imaging also reveals a formation in the abdominal cavity measuring up to 31.5 cm with uneven clear contours and a capsule up to 3 mm thick. The loops of the small and large intestines are mixed and separated by the formation. The stomach in the outlet section is displaced and compressed by the tumor. A picture of a non-organ retroperitoneal tumor of mixed structure is shown in Fig. 2.
Figure 2. Magnetic resonance imaging. The tumor has a heterogeneous structure and occupies most of the abdominal cavity.
Esophagogastroduodenoscopy determines the deformation of the body and antrum of the stomach due to external compression. There are no ulcerations of the mucous membrane. Mixed gastritis. Cardia failure.
X-ray examination of the stomach with a barium suspension revealed that the act of swallowing is not impaired, the esophagus is freely passable, the stomach is hook-shaped and has an inflection in the vault, the lower contour of the stomach is located 6 cm below the wings of the iliac bones (Fig. 3).
Figure 3. X-ray examination of the upper gastrointestinal tract with barium suspension. The antrum of the stomach and duodenum are spread out on the tumor. Evacuation from the stomach is not impaired. 1 - stomach filled with a radiopaque substance; 2 - the antrum of the stomach and duodenum are pushed back and spread out on the tumor. The contrast agent passes from the stomach in a thin stream into the duodenum. The antrum of the stomach and the duodenal bulb are spread out on the giant tumor and pushed anteriorly. The lumen of the stomach contains a small amount of liquid and mucus. The relief of the mucous membrane is not disturbed, the folds are thickened and directed parallel to the lesser curvature. Along the greater curvature, uneven serration is noted. Peristalsis is slightly slowed down, evacuation begins 2-3 minutes after a sip and occurs in small portions. The duodenal bulb is triangular in shape with elastic walls. The initial sections of the jejunum are without features. The X-ray picture is characteristic of a giant neoplasm of the stomach emanating from the wall of the antrum.
As a result of the examination, a diagnosis was made: giant nonepithelial tumor of the stomach (GIST?), cholelithiasis: chronic calculous cholecystitis.
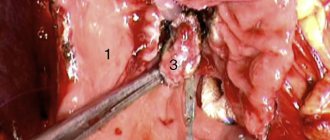
On July 20, 2010, the patient was operated on. A total midline laparotomy was performed. The peritoneum is smooth, shiny; no ascites. Most of the abdominal cavity is occupied by a huge cystic tumor with a whitish dense capsule, which is located anterior to the intestine. The tumor was easily removed from the abdominal cavity (Fig. 4, a).
Figure 4. Intraoperative picture. a — the tumor comes from the lesser curvature of the stomach. 1 - tumor, 2 - stomach spread out on the tumor.
There are no fusions with adjacent organs. During the audit it was found that the liver was of normal color and size, no metastases were detected. There is a stone in the gall bladder measuring 5x2 cm, there is no inflammation of the bladder. The loops of the small intestine and colon are not changed. The tumor originates from the lesser curvature of the lower third of the stomach. The duodenum is spread out along the right contour of the tumor; there is no invasion into the intestine or pancreas. The lymph nodes along the vessels of the celiac trunk are not enlarged. The uterus and its appendages are not changed. The situation corresponds to a giant GIST originating from the distal part of the stomach, cholelithiasis. The stomach and tumor were mobilized. A subtotal distal resection of the stomach was performed with immersion of the duodenal stump into a purse-string suture, suturing of the lesser curvature with UO-60 and peritonization with interrupted sutures. Gastroenteroanastomosis end to end in one row of sutures on a Py-loop formed from the jejunum (Py-loop length 60 cm). Cholecystectomy with ligation of the cystic duct and artery. The operation was completed by drainage of the subhepatic space.
Morphological examination: tumor with a diameter of 33 cm, weighing 5250 g (Fig. 4, b).
Figure 4. Intraoperative picture. b — macroscopic specimen of the removed tumor. Histological examination: spindle cell variant of GIST with an endophytic type of growth and tumor necrosis. An immunohistochemical study obtained the following results: CD117 (+++), CD34 (+++), smooth muscle actin (-), Ki-67 is 2%, mitoses are 2-3 in 50 fields of view. Polymerase chain reaction followed by sequencing revealed a mutation in the 11th exon of the KIT gene, which corresponds to the replacement of valine at position 559 with aspartic acid (V559D).
The postoperative period proceeded without complications. The patient was discharged from the hospital in satisfactory condition on the 10th day after surgery.
Discussion
Recently, a large number of works have been devoted to determining the prognosis of the course of the disease and treatment tactics for patients with GIST.
The prognosis of disease progression is based on the results of collective studies of more than 1000 patients treated in clinics in Poland [19], Taiwan [11], Japan [22], and the Mayo Clinic [9]. The main prognostic factors are: tumor size more than 10 cm; non-radical removal of the tumor, especially with damage to the integrity of the capsule; number of mitoses; KIT mutations; proliferation index; presence of nuclear polymorphism; tumor necrosis, involvement and ulceration of the gastrointestinal mucosa; germination of the serous membrane. Several schemes have been proposed for stratifying the risk of GIST recurrence, the most popular of which is the scheme proposed by H. Joensuu [12] (see table).
The results of large multicenter studies conducted in Sweden [18] and Modena [17] and at the US Armed Forces Institute of Pathology [16,15] have been published. However, today there is a limited amount of data on the criteria for determining the treatment algorithm for giant GISTs. A number of studies have been published showing the effectiveness of neoadjuvant targeted therapy. How important this is for patients in whom removal of giant GISTs is feasible remains unclear.
We believe that treatment of giant GISTs should begin with surgical removal of the tumor (unless this is associated with an unreasonably high perioperative risk) followed by targeted therapy with Gleevec. For locally advanced GISTs with invasion of great vessels and surrounding organs, it is advisable to consider neoadjuvant therapy.