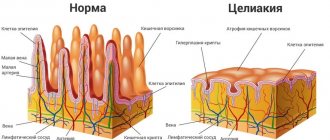
What is dysbiosis
A huge number of bacteria live and multiply in the human intestines. Normally, they do not cause harm to humans. Moreover, their presence is necessary for the normal functioning of the intestines and the body as a whole.
Dysbacteriosis is a phenomenon in which conditions are created in the intestines that enable the active reproduction of pathogenic microflora. In other words, this is a violation of the harmony of the intestinal microflora, which causes symptoms that are quite unpleasant for humans.
Diagnosis of the disease
In most cases, dysbiosis develops as a consequence of disorders on the part of other internal organs, which led to asthenization of the body and the formation of pathogenic flora. Therefore, at the initial stages, examination of other organs and systems of the child should be carried out to identify the cause of the disorder.
To establish the presence of dysbacteriosis, it is necessary to carry out a number of diagnostic procedures:
- scatological examination of a child’s stool - allows you to evaluate the enzymatic abilities of the digestive system, as well as confirm or deny the presence of an inflammatory process in the intestines;
- sowing of feces - determines only the number of opportunistic microorganisms, without their percentage ratio to other microbes;
- culture of urine and bile - carried out in advanced cases to confirm the generalization of the process and determine the predominant pathogen;
- inoculation of biopsy specimens from the mucous membrane of the duodenum, rectum and colon allows one to evaluate the parietal microflora.
Analysis for dysbacteriosis: explanation, norms - table
| Microflora | Normal indicators |
| Pathogenic enterobacteria | none |
| Escherichia coli with normal enzymatic activity (Escherichia) | 300–400 million/year |
| Escherichia coli with reduced enzymatic activity | to 10% |
| Lactose-negative enterobacteriaceae | up to 5% (103–106 million/year) |
| Hemolyzing Escherichia coli | absent |
| Coccal forms in the total amount of microbes | up to 25% (107 million/year) |
*In addition to the indicators indicated in the table, 90% of the flora should be lacto- and bifidobacteria.
How to collect and donate feces?
In order for the result of a stool test to be reliable, you should adhere to some rules for submitting biomaterial:
- the minimum required amount of feces is 5–10 g;
- collected in a sterile container, without urine;
- storage of material is unacceptable;
- three days before the test, the child should not receive new food;
- If you are taking medications, they must be discontinued on the day before collecting the material.
Is dysbiosis dangerous for an infant?
Is it necessary to talk about dysbiosis in children of the first year of life as a disease? This question remains open in modern medicine. Many experts consider it a special condition that needs correction.
Dysbacteriosis in infants manifests itself in many problems, such as constipation, diarrhea, allergic rash, etc. These symptoms, in fact, worry the baby. As you know, not only normal digestion, but also the baby’s overall well-being, as well as his immunity, depend on the condition of the intestines.
An imbalance of intestinal flora undermines the child’s body’s defenses and makes him vulnerable to viral infections.
The role of gastrointestinal microflora in child health
Immunity is responsible for the body's ability to resist disease. In newborns, an important role in its development is played by the microflora of the gastrointestinal tract, the main part of which is bifidobacteria. They do not allow pathogenic microorganisms to multiply, take part in the production of a number of essential vitamins and, most importantly, help strengthen the defenses.
Intestinal dysbiosis develops as a result of an imbalance of microflora
A breastfed baby receives bifidobacteria from the mother through milk, which contains not only microorganisms, but also stimulants for the growth of beneficial bacteria, antibodies and immune cells.
At the first contact between a woman and a baby, microflora is transferred. But the greatest influence on the further colonization of intestinal microorganisms is exerted by external factors.
Types of microflora of the gastrointestinal tract
The entire intestinal microbiota is conventionally divided into:
- the main one, which includes 90% of all types of microbes (mainly bifidobacteria and bacteroides);
- accompanying (lactobacteria, E. coli, enterococci) - up to 10% of the total composition of microbes;
- residual flora (klebsiella, citrobacter, proteus, tremors, clostridia, staphylococci) – less than 1%.
How to recognize dysbiosis in an infant
Observant parents can easily recognize the first symptoms on their own. The main signs of dysbiosis in children: diarrhea, anxiety, sleep problems, tearfulness, a tendency to develop allergic dermatitis, rash. The above symptoms are not a reason to make a diagnosis, but they should at least alert parents.
Characteristic symptoms of dysbiosis:
- flatulence;
- pale skin;
- lethargy;
- poor appetite;
- frequent attacks of colic;
- abdominal pain;
- dry skin;
- frequent manifestations of allergic dermatitis, rash;
- irritability;
- thrush in the mouth, stomatitis;
- constipation;
- diarrhea for more than 3 days;
- vomiting, nausea, frequent and profuse regurgitation;
- poor weight gain;
- green mucus in the baby's stool, blood impurities, foam.
It is worth noting that with the introduction of complementary foods, temporary changes in the consistency of the child’s stool, the frequency of bowel movements, the appearance of green mucus, diarrhea, and an allergic rash are possible. In most cases, such conditions do not require special treatment; everything will go away on its own. If not, you need to look for the causes of such disorders.
You should not self-medicate. If any symptoms occur, you should contact the doctor who is observing the child. After studying all the symptoms, the doctor will make a diagnosis.
What are the causes of intestinal microflora disturbances?
The causes of dysbiosis in infancy may be:
- maternal health problems that arise during pregnancy;
- pathologies during childbirth;
- various infections;
- physiological immaturity of the gastrointestinal tract of children under one year old;
- primary immunodeficiency;
- early feeding of children with dairy products, artificial feeding;
- late breastfeeding;
- use of hormonal drugs, antibiotics;
- stressful and/or unfavorable socio-psychological conditions in which the child finds himself.
Methods for diagnosing dysbiosis
Disturbances in the balance of intestinal microflora can be determined using stool analysis.
Before having stool tested, consult your doctor about the advisability of such a test. The interpretation of the test results should also be carried out by your doctor.
The following studies are carried out in laboratory conditions:
- Coprogram. Determining the degree of digestion of food by the intestines. It also helps to identify signs of inflammation in the gastrointestinal tract.
- Stool culture tank. Identification of the degree of formation of pathogenic intestinal flora.
- Stool culture for dysbacteriosis. Identification of the percentage ratio of the pathogenic and normal components of the microflora.
It would seem that what’s so difficult about collecting baby’s stool for analysis? For the results of the study to be reliable, this must be done correctly.
To properly collect stool for analysis, you should consider the following rules:
- Before collecting stool for analysis, the child should be washed and put on clean underwear; it is advisable to use a diaper, a homemade diaper (not disposable);
- long-term storage of collected material at room temperature is unacceptable;
- It is best if a sterile plastic container purchased from a pharmacy is used to collect stool;
- If the child eats mixtures containing prebiotics and probiotics before collecting the test material, they must be discontinued a few days before submitting stool for analysis.
Disorders of intestinal microbiocenosis in children and its correction
The most important role in the life of the human body is played by the intestinal microbiocenosis - the microecological system of the body, which developed in the process of phylogenetic development in the human digestive canal. The importance of microbial flora for a healthy person was first noticed in 1907 by I. I. Mechnikov. The process of interaction between microorganisms living in the intestines and the mucous membrane itself is quite complex and many monographs and scientific papers are devoted to its study.
The entire intestinal microflora can be divided into three groups: the main flora, among which the most interesting are bifidobacteria (Bifidobacterium bifidum, Bifidobacterium brevis, Bifidobacterium longum, Bifidobacterium adolescentis, etc.) and lactobacilli (Lactobacillus acidophilus, Lactobacillus fermentum, Lactobacillus brevis, Lactobacillus lactis, etc. .), accompanying flora, represented mainly by E. coli with typical biological properties and enterococci (Enterococcus fecalis, Enterococcus faecium), and residual flora, which is represented by opportunistic bacteria of the enterobacteria family: Klebsiella, Citrobacter, Proteus, etc., staphylococci (Staphylococcus epidermidis, Staphylococcus saprophyticus) and yeast-like fungi.
One of the main functions of normal microflora is protective, since human symbiont bacteria have pronounced antagonistic activity towards pathogenic and opportunistic microorganisms. For example, bifidobacteria form lactic, acetic, formic and succinic acids during their life, which reduces the pH of the intestinal environment and prevents its colonization by foreign microorganisms that have come here from the outside. During the fermentation of lactic acid, lactobacilli form antibiotic substances - lactolin, lactocidin, acidophilus. Full-fledged E. coli is capable of synthesizing colicins and microcins - specific proteins with antibacterial activity. Thus, representatives of the main and accompanying intestinal microflora inhibit the growth and reproduction of putrefactive and pathogenic microorganisms - E. coli with atypical biological properties, Klebsiella, Proteus, some types of Salmonella and Shigella, Staphylococcus aureus.
The presence of microbial associations in the intestine determines the state of the synthetic function of liver cells through participation in the hepatic-intestinal circulation of the most important components of bile - bile salts, cholesterol and bile pigments.
The participation of normal intestinal microflora in the metabolic processes of the human body is determined, on the one hand, by the utilization of undigested food compounds by intestinal microorganisms and the inactivation of biologically active substances released with digestive juices, and on the other hand, by the synthesis by representatives of the normal intestinal flora of B vitamins, vitamin K, nicotinic and folic acid, various biologically active compounds: estrogens, promazine, morphine, colchicine, diestilbestrene, digoxin, etc.
Another of the most important functions of the microflora of the human body is participation in the formation of the immunobiological reactivity of the macroorganism. It has been shown that as a result of antigenic stimulation of the immune system by autoflora, a common pool of immunoglobulins is created and maintained in the human body.
It is known that intestinal enterocytes, whose main function is to absorb nutrients from the intestinal lumen, do not always receive adequate nutrition from the body itself, through the basement membrane to which they are fixed. This is explained by the fact that infusion processes in intestinal epithelial cells seem to proceed in only one direction - from the top of the enterocytes to the basement membrane, and not vice versa. So the enterocytes have to “get food” for themselves also from the intestinal lumen. This nutritional substance for epithelial cells, called butyrate, consists of various waste products of the symbiont flora living in the intestinal lumen, which is a mixture of short-chain fatty and volatile acids, monosaccharides and lipids.
When the symbiont flora is suppressed by opportunistic and pathogenic bacteria, butyrate production decreases or stops altogether, enterocytes in a state of nutritional deficiency atrophy and digestive processes are disrupted. There are works describing mechanical damage to intestinal villi under such conditions by opportunistic Escherichia coli. Escherichia literally “gnaws” on enterocytes weakened due to nutritional deficiency.
Thus, normal microflora with its specific functions - protective, metabolic and immuno-inducing, nutritional - determines the microbial ecology of the digestive tract and participates in maintaining the homeostasis of the macroorganism. Violation of any of the functions leads to disruption of various types of metabolism, the occurrence of micronutrient deficiency: vitamins, microelements, minerals in the human body, and a decrease in its immune status.
Violations of the composition and quantitative ratio in the intestinal microbiocenosis arise from a variety of reasons: the nature of nutrition, age, antibacterial, hormonal or radiation therapy, the presence of chronic diseases of the gastrointestinal tract, altered immunobiological reactivity of the body and environmental conditions and occur long before clinical manifestations - they serve as a harbinger of deviations in the clinical and physiological status of the body. These changes in the normal microflora are called dysbiosis or dysbiosis, which over time manifests itself with clinical local symptoms, and then general disorders that aggravate the course of various human diseases and complicate their treatment. The predominance of growth of any representatives of the intestinal microbial flora, which occurs due to the circumstances listed above, is called bacterial overgrowth syndrome (SIBO) and is one of the manifestations of dysbiosis. Therefore, along with treating a person for a particular disease, it is necessary to improve his intestines.
To characterize intestinal dysbiosis in different years, various classifications have been proposed, taking into account the type of microbial flora, type of disorder, severity and clinical forms, etc., but none of the currently known classifications can fully satisfy the doctor when solving practical problems of normalization and correction of intestinal microbiocenosis and rationally construct the treatment and prevention of dysbiosis in patients. We used the classification by S. D. Mitrokhin (1998), which allows us to characterize the severity of colon dysbiosis from the standpoint of optimizing modern approaches to the treatment and prevention of dysbiosis.
Microecological classification of the severity of intestinal dysbiosis (S. D. Mitrokhin, 1998)
I degree of severity - the total number of E. coli is increased or decreased. There are no E. coli with atypical biological properties. The number of bifidobacteria and lactobacilli was not changed. Changes in the general indicators of the microbial metabolite passport of feces are characteristic only in relation to the pool of volatile fatty acids, the content of phenylpropionic acid, skatole and methylamine. The total content of volatile fatty acids in patients with the first degree of severity of dysbiosis may be less or greater than that in healthy individuals. Skatole content will be reduced. On the contrary, the content of phenylacetic acid and methylamine will be increased. Changes in the specific gravity of oxaloacetic acid in the profile of other carboxylic acids (DCA) will be noted. Dysbacteriosis is latent, compensated, intestinal dysfunctions are not recorded.
II degree of severity - the number of bifidobacteria and lactobacilli is slightly reduced. Quantitative and qualitative (appearance of forms with atypical biological properties) changes in E. coli are observed. Opportunistic intestinal microorganisms are sown in moderate quantities. There are changes in both general and specific indicators of the microbial metabolite passport of feces. Which is reflected in a decrease in the amount of excretion of phenolic compounds in feces: p-cresol and indole. The amount of skatole in feces decreases tenfold. The opposite picture is observed for phenylpropionic acid, the amount of which is an order of magnitude higher than that in healthy individuals. The profile of phenolic compounds (PCs) also changes: the specific gravity of indole increases by more than 2 times, the specific gravity of p-cresol has decreased slightly, and the specific gravity of skatole has decreased by more than ten times. In general, fecal excretion of carbonic and aromatic amino acids, phenolic and indole compounds (with the exception of phenylalanine) is reduced in grade II dysbiosis. Excretion of histamine and serotonin in feces decreases. The amine profile has been changed: the specific gravity of histamine and serotonin is lower, the specific gravity of the representative of simple amines, methylamine, is higher. Dysbacteriosis is local (local), subcompensated, intestinal dysfunctions, as a rule, are not observed.
III degree of severity - a significant decrease in the number of bifidobacteria (105–106) combined with a decrease in the number of lactobacilli and a sharp change in the typical properties of Escherichia coli (a significant predominance of hemolytic, lactose-negative forms). A significant increase in the number of opportunistic bacteria with pathogenic properties (hemolytic forms) and pathogenic yeast-like fungi (genus Candida, Geotrichum, etc.). Characterized by even more pronounced changes in both general and specific indicators of the microbial metabolite passport of feces. The amount of excretion with feces of phenolic compounds: p-cresol and indole is reduced. Skatole is practically absent in feces. On the contrary, the content of phenylpropionic acid in feces increases sharply. The PS profile changes in such a way that the specific gravity of indole increases significantly and the specific gravity of p-cresol decreases significantly. The amount of amine excretion changes significantly: the content of histamine and serotonin in feces may be higher or lower compared to the norm (depending on the nosological form of the underlying disease). The content of carboxylic acids in the feces of patients with stage III dysbiosis changes as follows: the pool of volatile fatty acids sharply decreases, oxaloacetic acid is practically not detected, and excretion of alpha-ketoglutaric acid with feces increases significantly. Changed volatile fatty acid profile. In case of stool disorders such as diarrhea, the specific gravity of acetic acid is lower, the specific gravity of propionic and butyric acids, on the contrary, is increased; with constipation, the opposite picture is observed. There is a decrease or increase in the specific gravity of lactic acid and similar changes in the specific gravity of alpha-ketoglutaric acids in the DKK profile. Dysbacteriosis is local (local), decompensated, intestinal dysfunctions are usually observed.
IV degree of severity - a sharp decrease or absence of bifidobacteria, a significant decrease in the number of lactobacilli, a sharp decrease in the number or absence of E. coli with typical properties, a significant increase in the number of both obligate and facultative species (not normally found) of intestinal bacteria and yeast-like fungi with pathogenic properties . Pathogenic intestinal bacteria (Salmonella, Shigella, Yersinia) are detected. Qualitative changes in the microbial metabolite passport remain the same as in the third degree, but their quantitative characteristics are even more changed, characterized by a deep imbalance of the biochemical regulatory mechanisms of the microbial ecosystem, combined with a similar imbalance of the intestinal microbial infrastructure. Dysbacteriosis is widespread (with bacteremia), decompensated (with the threat of generalization of infection, sepsis or septicopyemia), pronounced intestinal dysfunctions are observed.
Doctors from all over the world have been treating dysbiosis for a long time. As a result of many years of painstaking search for the most effective measures and methods, a large number of medications and biologically active additives have now been formed, which to one degree or another affect the state of the microflora and are used to restore the disturbed balance of the intestinal eco-structure.
The wide variety of drugs on the Russian market, the need for a rational choice of therapeutic effects and their combinations convincingly indicate the need for some systematization and schematization of the treatment of intestinal dysbiosis, without excluding experience and creativity in the work of the attending physician and an individual approach to the correction and prevention of intestinal dysbiosis.
Currently, the following basic principles of combined correction of intestinal microecological imbalance have been developed:
- correction of the motor-secretory function of the gastrointestinal tract, including enzyme therapy, multivitamin therapy, prescription of antispasmodics, antidiarrheal, choleretic and drugs that stimulate regenerative processes;
- enterosorption and enteroprotection, which consists in the administration of enterosorbents that remove both pathogenic microorganisms and their metabolic products from the body;
- selective decontamination of pathogenic and conditionally pathogenic intestinal microflora, carried out on the basis of the administration of bacteriophages, intestinal antiseptics, phytoncides and antibacterial drugs;
- correction of normal intestinal microflora using various probiotics;
- functional nutrition, which consists of consuming fermented milk and other products containing bifidobacteria as an active ingredient (fruit and vegetable juices, chewing gum, ice cream, confectionery, salads, cheeses, sour cream, kefir, butter, cream, cottage cheese, desserts and etc.). Currently, bifidobacteria are included in more than 70 different food products.
The following points are also extremely important elements in the treatment and prevention of intestinal dysbiosis:
- sanitary and educational work with patients and their relatives about the role of microflora and the need for its correction;
- long-term cooperation between doctor and patient;
- adherence to dietary regimen and functional nutrition;
- the use of medicinal and non-medicinal methods of treatment and prevention of intestinal dysbiosis.
When constructing tactics for rational, individual treatment and prevention of intestinal dysbiosis, especially in children of different ages, in whom the processes of forming their own ecosystem continue until 10–12 years of age, the doctor needs to solve the following problems:
- normalization of intestinal function (the first principle of combination therapy);
- creating conditions conducive to more favorable development of the body’s own flora (autoflora) (the second principle of combination therapy);
- sanitation of the intestines from pathogenic and opportunistic flora (the third principle of combination therapy);
- long-term intermittent treatment (pulse therapy) for III-IV severity of dysbacteriosis for at least 6 months (the fourth principle of combination therapy);
- preservation and maintenance of intestinal microbial ecology (fifth principle of combination therapy).
To normalize intestinal function, agents are used and individually selected, taking into account dysfunctions of the colon and the characteristics of the action of drugs with the most likely effect on the cause of the existing intestinal dysfunction (constipation, diarrhea) and adsorbing properties, as well as enzymatic deficiency. Recently, much attention has been paid to the problems of dysbiosis, excessive bacterial growth caused by a variety of factors - disruption of the macro- and microecology of the intestine due to antibiotic therapy, chemotherapy, food poisoning, etc. These works dwell in some detail on the etiopathogenetic mechanisms of the development of dysbiosis and its consequences and on treatment methods using intestinal antiseptics, antibiotics and various mono- or multi-strain probiotics. Probiotics include biological active agents containing colonies of live or lyophilized microorganisms, the main function of which is replacement, that is, when taking them, it is necessary to replace pathogenic and conditionally pathogenic flora with strains of specially bred cultures of bifido-, lacto- and other bacteria, similar in their properties to strains living in the human intestine. The topic of replacement therapy is especially relevant for children of different ages. As already mentioned, the intestinal microflora begins to form from the first day of a child’s life and ends, according to various authors, by 10–12 years. The ratios of different groups of bacteria formed by this age are individual for each person and remain in his intestines throughout almost his entire life with slight fluctuations depending on external and internal (age-related) factors. And if, for some reason, an adult’s ecosystem is disrupted, then in this case it is possible to prescribe broad-spectrum antibiotics to sick people with less caution, followed by a course of probiotics. The point of such treatment is to destroy pathogenic microorganisms from the surface of the intestine. Of course, during this period the symbiont microflora also suffers significantly. However, under the guise of probiotics, which, as a rule, are prescribed after the end of antibiotic therapy (and modern types of probiotics that have strains resistant to some antibiotics, and during antibiotic therapy) in the intestine sooner or later, depending on the individual characteristics of the organism, the surface of the mucous membrane the intestines will again be populated by those first “native” strains of symbionts that settled in the human body during the formation of the biocenosis in the first decade of the life of the macroorganism. Some of their representatives will survive in any case, even under the influence of antibiotic therapy, and will subsequently give rise to new colonies, which, under the “cover” of probiotic strains, will develop and colonize the entire surface of the mucous membrane. And probiotic strains, which are migratory flora, will leave the body. The transient nature of the presence of probiotic microorganisms in the intestine has been confirmed by studies that assessed the duration of their detection in stool. According to the results of these studies, these strains were not detected in the stool already 1–4 weeks after the end of taking the probiotic agent (Ciorba, 2010). The situation is different with children whose own intestinal ecosystem has not yet been fully formed, although, of course, the main balance of microflora is already determined by the end of the first year of life. Prescribing antibiotics to children for intestinal sanitation can have a detrimental effect on the further formation of normal microflora, and in this case, probiotics, even containing normal bifidobacteria and lactobacilli and symbiont enterococci, can create competition for the “native” intestinal strains. Prescribing antibacterial therapy is fraught, especially in children, with the possible rapid development of pseudomembranous colitis with a predominance of Clostridium difficile development. In this case, if possible, it is better for children to be prescribed not antibiotics, but intestinal antiseptics, for example, the nitrofuran series, which have a “softer” effect on the microflora, suppressing the growth of predominantly aerobic opportunistic and pathogenic strains of microorganisms. But for the speedy restoration of the microecosphere, these antiseptics, even non-aggressive and mildly acting ones, are not always enough. Currently, special sets of strains have been developed for children, included in the prescriptions of various drugs, which are aimed at quickly restoring the child’s natural microflora. However, they are not an ideal means for stimulating the growth of their own, not yet strong colonies of symbiont microorganisms. In this case, it is precisely to stimulate the growth of the symbiont flora of bifidobacteria, lactobacilli and enterococci, and primarily to stimulate the growth of primary, “naturally” colonized strains, that it is recommended to use products that are an extract of the vital activity of the symbiont flora. That is, in fact, the prototype of butyrate, which is a nutrient medium for enterocytes. Such drugs include Hilak forte, which is a metabolic probiotic. The drug is a transparent light yellow or yellow-brown liquid with a characteristic sour odor. The drug contains germ-free aqueous substrates of biosynthetic products of bacterial metabolism of four types of microorganisms present in the normal intestinal microflora: Escherichia coli, Enterococcus faecalis, Lactobacillus acidophilus and Lactobacillus helveticus. Due to the presence in the preparation of natural waste products of bacteria, similar to those present in the intestinal lumen with normal microflora, it helps maintain the physiological functions of the intestinal mucosa and its microbial ecology. Creates favorable conditions that provide nutrition to weakened or damaged enterocytes, thereby restoring the cytomucoprotective ability of the mucous membrane. Accelerates the development of normal intestinal microflora, creating an optimal ecological environment with a certain ratio of acids. The drug is prescribed to children by taking 20–40 drops 3 times a day before or during meals. For infants, the dosage is 15–30 drops 3 times a day. An intermittent rhythm of 10–15 days with breaks of 1–2 months is recommended.
It is possible that when taking the drug, not only and not so much the acidity of the environment in the small intestine is restored (since the dose of the drug is very small), but some trigger mechanisms are launched that regulate the process of cytomucoprotection and affect the microecological system in the intestine. This is one of the assumptions, scientific research on which continues until recently. However, there is clinical effectiveness when taking Hilak Forte in the case of the development of dysbiosis in adults and children of different ages. When taking this drug, the microecological balance in the intestines is quickly restored and the clinical manifestations accompanying dysbiosis disappear - abdominal pain, flatulence and constipation, as evidenced by clinical studies in different countries.
It is known that an important role in the positive effect of probiotic microorganisms is played not by the bacterial cells themselves, but by their metabolites (N. I. Ursova, 2013). In this regard, Hilak Forte may be more preferable than lyophilized forms of various probiotic preparations, which require 8–10 hours to activate and begin producing metabolic products (S. K. Adzhigaitkanova, 2007). This issue becomes especially important in the case of diarrhea, which often accompanies microflora disorders. The fact is that the time of normal transit of contents through the intestines in both adults and children allows lyophilized probiotics to undergo activation. However, general intestinal transit during diarrhea can be significantly accelerated. Thus, according to the results of a study in children 3–18 months with diarrhea, the Total gut transit time was only 5 hours (Roy, 1991).
An important issue is the correction of microflora disorders when taking antibacterial therapy. Preparations based on metabolites of beneficial bacteria, such as Hilak forte, do not contain living microorganisms. Therefore, their use in conjunction with antibiotics may be more preferable, since the latter in no way directly reduce their effectiveness.
In any case, in order not to disturb the fragile microecological structure of the intestine or restore it after any aggressive influences, it is necessary to use the entire arsenal of means currently available in medicine in general and in pediatric gastroenterology in particular, combining the use of probiotics along with , promoting the rapid adaptation of colonies of symbiont bacteria on the intestinal mucosa - products of the natural metabolism of microorganisms, which include Hilak forte.
The table shows biochemical criteria for the state of the intestinal microbial ecology obtained from examining practically healthy people - blood donors [37].
Literature
- Kuvaeva I. B., Ladodo K. S. Microecological and immune disorders in children: dietary correction. M., 1991.
- Zaprudnov A. M., Mazankova L. N. Microbial flora of the intestine and probiotics. M., 1999.
- Korshunov V.M., Volodin N.N., Efimov B.A. et al. Microecology of the gastrointestinal tract. Correction of microflora in intestinal dysbiosis. M., 1999.
- Adzhigaitkanova S.K. Approaches to drug treatment of intestinal dysbiosis // Diseases of the digestive organs. RMJ application. 2007. No. 2. P. 73–76.
- Ursova N. I., Rimarchuk G. V., Shcheplyagina L. A., Savitskaya K. I. Modern methods for correcting intestinal dysbiosis in children (textbook). M., 2000.
- Ursova N.I. Current and unresolved problems of probiotic therapy // Treating Doctor 2013. No. 8. P. 60–65.
- Petrovskaya V. G., Marko O. P. Human microflora in normal and pathological conditions. M., 1976.
- Timofeeva G. A., Tsinzerling A. V. Acute intestinal infections in children. L., 1984.
- Pokrovsky V. I., Gordienko S. P., Litvinov V. I. (eds.) Immunology of the infectious process. M., 1994.
- Shenderov B. A. Medical microbial ecology and functional nutrition. M., 1998.
- Shadrin S. A. Intestinal dysbiosis in children. Krasnodar, 1999.
- Mukhina Yu. G. Features of the microecology of the digestive tract in children. On the problem of dysbacteriosis // Children's Hospital. 2000, no. 2.
- Rosebury T. Microorganisms indigenous to man. NY, 1962.
- Collins MD Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut // Am. J. Clin. Nutr. 1999; 69(Suppl.): S1052–1057.
- Vanderhoof JA, Whitney DB, Antonson DL et al. Lactobacillus GG in prevention of antibiotic-associated diarrhea in children // J. Pediatr. 1999; 135:564–568.
- Mitrokhin S. D., Shcherbakov P. L., Bredikhina N. A., Elizavetina G. A., Ivanikov I. O., Minushkin O. N. Treatment and prevention of intestinal dysbiosis (dysbiosis) at the present stage. Practical recommendations. M., 1999, 48 p.
- Ursova N. I. Intestinal dysbacteriosis in childhood: innovations in diagnosis, correction, prevention. M., 2013. 328 p.
- Parfenov A.I. Enterology. M.: MIA, 2009. 880 p.
- Rambaud J.-C. et al. Gut Microflora. Paris: John Libbey eurotext, 2006. P. 247.
- Amandine Everarda, Clara Belzerb, Lucie Geurtsa et. al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity www.pnas.org/cgi/doi/10.1073/pnas.1219451110 PNAS Early Edition.
- Emmanuelle Le Chatelier, Trine Nielsen, Junjie Qin et al. Richness of human gut microbiome correlates with metabolic markers // 038/nature12506.
- Shuiming Xiao, Na Fei, Xiaoyan Pang et al. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome // FEMS Microbiol& Ecol. 2013, 1–11.
- Treatment and prevention of intestinal dysbiosis (dysbiosis) at the present stage. Practical recommendations.
- Prakash S., Tomaro-Duchesneau C., Saha S., Cantor A. The gut microbiota and human health with an emphasis on the use of microencapsulated bacterial cells // J. Biomed. Biotechnol. 2011: 981214. [email protected] Jul 2: 981214.
- Reid G., Gaudier E., Guarner F. et al. Responders and non-responders to probiotic interventions: how can we improve the odds? // Gut Microbes. 2010; 1:200–204.
- Rosenberg E., Zilber-Rosenberg I. Symbiosis and development: the hologenome concept // Birth Defects Res.C. Embryo.Today. 2011; 93:56–66.
- Roy SK et al. Persistent diarrhea: total gut transit time and its relationship with nutrient absorption and clinical response // J Pediatr Gastroenterol Nutr. 1991, Nov; 13 (4): 409–414.
- Tlaskalova-Hogenova H., Stepankova R., Kozakova H. et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases // Cell Mol. Immunol. 2011; 8: 110–120.
- Xue H., Sawyer MB, Wischmeyer PE, Baracos VE Nutrition modulation of gastrointestinal toxicity related to cancer chemotherapy: from preclinical findings to clinical strategy // JPEN J. Parenter.Enteral Nutr. 2011; 35: 74–90.
- Whelan K., Schneider SM Mechanisms, prevention, and management of diarrhea in enteral nutrition // Curr. Opin. Gastroenterol. 2011; 27: 152–159.
- Delzenne N., Cherbut C., Neyrinck A. Prebiotics: actual and potential effects in inflammatory and malignant colonic diseases // Curr. Opin. Clin. Nutr. Metab Care. 2003; 6:581–586.
- Cabre E., Gassull MA Nutritional and metabolic issues in inflammatory bowel disease // Curr. Opin. Clin. Nutr. Metab Care. 2003; 6:569–576.
- Meier R., Burri E., Steuerwald M. The role of nutrition in diarrhoea syndromes // Curr. Opin. Clin. Nutr. Metab Care. 2003; 6:563–567.
- Matthew Ciorba. A Gastroenterologist's Guide to Probiotics // Clin Gastroenterol Hepatol. 2012; 10(9):960–968.
- Reid G., Sanders ME, Gaskins HR et al. New scientific paradigms for probiotics and prebiotics // J. Clin. Gastroenterol. 2003; 37: 105–118.
- Holmes S. Are probiotics and other functional foods the medicines of the future? // Prof. Nurse. 2003; 18: 627–630.
- Mitrokhin S. D., Ardatskaya M. D., Nikushkin E. V. et al. Complex diagnosis, treatment and prevention of intestinal dysbiosis (dysbiosis) in the clinic of internal diseases. Methodological recommendations/Ed. O. N. Minushkina. M.: Medical Center of the Presidential Administration of the Russian Federation, 1997. 45 p.
P. L. Shcherbakov, Doctor of Medical Sciences, Professor
GBUZ MKNTs DZM, Moscow
Contact Information
Treatment of dysbiosis in infants
Parents should not be afraid of this diagnosis, because modern medicine knows how to treat dysbiosis in infants.
It will be much easier and faster to cure dysbiosis in infants by identifying the causes of its occurrence. Parents who discover signs of a violation of the intestinal microflora in their children should immediately contact their family doctor. It is he who will prescribe the correct treatment in your particular case.
It is the doctor (and not you yourself) who must determine the causes and give you practical recommendations for eliminating them.
Tactics for treating dysbacteriosis
As a rule, treatment of dysbiosis is quite long. Special medications containing live lacto- and bifidobacteria are required. The treatment regimen is prescribed by the doctor, who also observes the child and adjusts the therapy aimed at restoring normal intestinal microflora. For example, a course of taking Linex can last from 5 to 7 days, after which a noticeable improvement is usually observed. If necessary, the doctor will adjust the treatment regimen.
First, the doctor will prescribe drugs that kill pathogenic bacteria. At the same time, sorbents are prescribed to remove toxins from the body. And in the future, treatment tactics will be aimed at populating the intestines with beneficial lacto- and bifidobacteria with the help of medications and milk formulas. To maintain intestinal health, it is recommended that all family members maintain a healthy lifestyle in all its manifestations.
If the child above is still breastfed, a necessary condition is the normalization of the feeding mother's diet. You should consume more fermented milk products, completely abandon the so-called food “garbage”: sausages, sausages, mayonnaise, ketchup, packaged juices, carbonated drinks, chips, etc.
If you are already introducing complementary foods, you should definitely give your baby fermented milk products every day.
Treatment of dysbiosis with medications
To treat dysbiosis in modern medical practice, lactulose preparations are widely used under various commercial names. They are absolutely safe for the health of children and are well tolerated by them.
A common practice is to prescribe a course of treatment with the drug "Linex", which contains bacteria that restore normal intestinal microflora.
The drug "Linex" is widely used to treat dysbiosis in children under 2 years of age. When using it to treat newborns and infants, you first need to open the capsule, then mix the contents with a small amount of water. Linex is also used to prevent dysbiosis during antibiotic treatment.
Most often, the following are used to treat dysbiosis in infants: bacteriophages, probiotics, Acipol, Linex, Enterol, bifidumbacterin, bifiform and others.
These preparations contain beneficial bacteria, microorganisms to restore positive intestinal microflora, as well as vitamins necessary to maintain immunity.
What are the reasons
The appearance of dysbiosis in infants can be caused by a number of reasons. Depending on what exactly caused the disease, primary and secondary dysbacteriosis can be distinguished.
Most often, this diagnosis is heard by parents of children who have not yet turned 1 year old, as well as those who were born prematurely, so their intestines have not yet fully formed. Signs of dysbiosis in children (primary) can be caused by the following reasons:
- The baby has been on artificial feeding since birth or was switched to it too early.
- During the breastfeeding period, my mother took a course of antibiotics and hormonal drugs.
As for the reasons for the appearance of secondary dysbiosis in a child, they can be as follows:
- Disruption of the gastrointestinal tract.
- The infant is taking medications that contain an antibiotic.
- Enzymes responsible for digestion are produced in small quantities.
- The intestines are physiologically immature.
- The baby has poor nutrition and complementary foods have been introduced into the diet ahead of time.
- Bacteria and parasites have entered the body and colonize the microflora with pathogenic microorganisms.
- During childbirth, the baby suffered trauma.
- The infection entered the baby's body during breastfeeding from a mother suffering from mastitis.
- Staphylococcus aureus.
Having found out the reasons why symptoms of dysbiosis appear in children under 1 year of age, timely, correct treatment should follow.
It is aimed not only at eliminating the cause, but also at restoring the intestinal microflora. Therapy may take a long time, but it is important to complete it, because the baby’s weakened immune system may be susceptible to this disease again.
Prevention of dysbacteriosis in infants
When there is a risk of intestinal dysbiosis, it is advisable to take preventive measures.
Having prescribed a course of antibiotic treatment to prevent dysbiosis, doctors usually simultaneously prescribe drugs that restore intestinal microflora (Linex, lactulose syrup, etc.).
Doctors often prescribe the drug “Linex” to prevent dysbiosis from the first days of a baby’s life. This is especially true for bottle-fed children. This medicine is usually well tolerated; side effects in rare cases may include hypersensitivity reactions (rash, diarrhea, etc.), which are not dangerous. In such cases, you should consult your doctor before continuing treatment with this drug.
An overdose of the drug "Linex" is possible if the doses specified in the instructions for the corresponding age are exceeded. You should be careful.
Basic measures to prevent dysbiosis in infants:
- Early breastfeeding. The first drops of colostrum entering the baby’s mouth create the most powerful defense for his body, populating the intestines with beneficial bacteria.
- Breast-feeding. But parents of bottle-fed babies should not despair. The variety of modern milk formulas allows you to select appropriate treatment regimens.
- Balanced healthy diet for a nursing mother.
- Caring for the health of parents at the planning stage and during pregnancy. A consultation with a gynecologist before conception will not be superfluous. While pregnant, it is necessary to undergo the necessary examinations in time (before the moment of birth) and, if necessary, carry out an appropriate course of treatment.
- A healthy lifestyle for parents and children in all its manifestations.