GastrituNet.ru » Stomach diseases » Structure » Physiology
There are substances in the human body that perform important digestive functions. One of the components is hydrochloric acid in the stomach. This is a product secreted by the main glands of the fundus. A change in its homeostasis leads to a deterioration in the patient’s condition and disruption of his quality of life.
- 1 What is hydrochloric acid and how is it produced?
- 2 Functions of hydrochloric acid
- 3 Protein denaturation
- 4 Acidity as an indicator of stomach condition
- 5 Methods for studying the acidity of gastric juice
- 6 Acid-dependent diseases of the gastrointestinal tract
- 7 Useful video
- 8 Signs, causes and consequences of increased acidity
- 9 Signs, causes and consequences of low pH
- 10 Methods for pH normalization
What is hydrochloric acid and how is it produced?
In order to fully understand the functional role of hydrochloric acid in the stomach, it is necessary to study the entire process.
Digestion begins when the thought of food arises and its smell is felt. Receptors are activated, the central nervous system centers are activated, and information about the upcoming act of eating is provided. As a result, the fundic glands become aware of the need for gastric juice. This is the first phase of secretion. The stomach prepares for food intake by secreting a meager amount of enzymes.
After eating food, these impulses intensify, and much more secretion is released. Parietal cells, thanks to chemoreceptors, capture information about the reaction of the environment and regulate it by releasing acid. The second phase of secretion is the most basic and depends directly on the release of gastrin. It stimulates glandular cells and provokes maximum release of hydrogen chloride during the act of eating.
The final phase occurs thanks to somatostatin. It is released into the stomach after a signal that food has entered the duodenum. The stretching of the stomach and the pressure on the receptors becomes less, the need for secretion of gastric juice decreases. Somatostatin deactivates the cells of the fundus of the stomach, and acid secretion decreases to a minimum level. Once in the duodenum, the pH becomes alkaline due to neutralization by bile.
Why doesn't stomach acid eat away at the stomach itself?
Amazing thing.
Our stomach, digesting and dissolving any food with gastric juice, leaves the stomach itself intact. Moreover, animal stomachs are used in the cuisines of many countries. And our stomach digests the stomachs of others, but does not suffer itself. Why? To do this, you need to understand how the stomach works. Food is digested with the help of enzymes contained in gastric juice. One of the components is hydrochloric acid.
When we don't eat, gastric juice is produced in very small quantities and is slightly acidic. But when eating, it begins to be produced in large quantities (up to two liters per day) and with high acidity. Hydrochloric acid dissolves the food that enters the body and simultaneously kills all the bacteria contained in it.
Food begins to be digested almost immediately as soon as it is in the stomach. Over time, food turns into a homogeneous liquid mass called chyme. Next, the chyme enters the duodenum.
The stomach itself is protected from gastric juice by the so-called mucus-bicarbonate barrier. The walls of the stomach are covered with epithelium, a layer of cells. These cells produce a layer of mucus that is evenly distributed throughout the inside of the stomach. Mucus is produced simultaneously with the production of gastric juice. The thickness of this mucus can reach one millimeter. This mucus reliably protects the walls from corrosion.
At the same time, the walls of the stomach themselves produce an additional protective barrier - sodium bicarbonate, or baking soda. Baking soda can effectively neutralize acid and is often used in various medications to reduce stomach acidity.
This double protection allows the stomach to actively digest food and not be afraid of corroding itself. But sometimes our body malfunctions, and the reliability of the protective barrier is broken. As a result, a person begins to suffer from stomach pain - gastritis or ulcers, which are pathological changes in the gastric mucosa. This is a potentially dangerous organ condition that requires treatment.
If you like the article, give it a thumbs up! It’s not difficult for you, but it’s nice for us!
Subscribe to the channel, tell us about it on social networks, and we will try not to lose face)
Source
Functions of hydrochloric acid
Hydrogen chloride converts pepsinogen into an active compound necessary for the digestion of chyme. Its function is to break down proteins into short amino acid chains. The enzyme requires an optimal acidic environment for normal metabolism.
The pH in the stomach varies. If a person has not eaten for a long time, the pH of gastric juice is usually around 4. When food enters the stomach, HCl production increases and the pH may drop to 1 or 2, in very acidic conditions. The acid not only provides a suitable environment for pepsin, but also kills many potentially dangerous microbes that enter the stomach with food. It also regulates the optimal pH in the stomach for the development of microflora on the mucous membrane.
The digestive function of hydrochloride compounds is the ability to break down protein molecules into amino acids and denature proteins. When dairy products enter the stomach, they curdle and casein is formed along with pepsins and chemosins.
How is production regulated?
As food moves forward, it enters first the acid-forming and then the acid-neutralizing zones of the stomach. They are separated by an intermediary zone, which corresponds to the transition of the fundus to the antrum. Therefore, a slightly acidic environment with a pH level of 4.0-6.0 is replaced by a strongly acidic environment, where the pH is less than 3.0.
The main acid-forming function is performed by hydrochloric acid in the stomach. Correction of acid formation is carried out by hormonal and biologically active components as the chyme moves. The fundic glands of the body and antrum secrete acid. The process is regulated with the help of histamine. This substance is produced when the walls of the organ are stretched by food. Gradually, the concentration of gastric juice increases, which leads to the activation of somatostatin. It blocks the secretion of hydrochloric acid. Another neutralizing mechanism is bicarbonates, which make food alkaline. An optimal environment with low acidity is needed for the reflex opening of the sphincter and the creation of normal peristalsis in the stomach and duodenum.
Hypersecretion of hydrochloric acid causes retention of chyme in the stomach; its deficiency leads to its premature entry into the intestine. The process of fermentation and absorption of nutritional components is disrupted.
Causes of hypersecretion
Increased acidity occurs due to exposure to negative factors. The pathology is accompanied by inflammation of the mucous membrane when gastric juice is produced in excess.
The condition is accompanied by unpleasant symptoms characteristic of hyperacid gastritis.
- pain while eating;
- heartburn and belching with a hint of sour;
- nausea and vomiting;
- flatulence;
- bowel dysfunction in the form of constipation.
The main reason is identified when the organ secretes hydrochloric acid in large quantities. This is Helicobacter pylori infection. The pathogenic bacterium is resistant to the acidic environment in the stomach. It causes an immune inflammatory reaction of the mucous membrane, secretes enzymes that break down mucus, and produces urease, which ferments urea to form ammonia. As a result, parietal cells are modified, which leads to an excess of secretions and an increased risk of cancer.
Other provoking factors include:
- stress, psycho-emotional stress;
- violation of the nature of nutrition and regime;
- chemical irritants, including some medications;
- genetic predisposition;
- parasitic diseases;
- bad habits - alcohol and smoking tobacco products.
Reasons for shortage
A decrease in the concentration of gastric juice most often occurs against the background of chronic gastritis. The pathogenic effect of unfavorable factors over a long period of time leads to gradual atrophy of the mucous membrane. Another reason for decreased secretion release is autoimmune damage to cellular structures. Therefore, there is a lack of hydrochloric acid in the stomach, which is manifested by the following symptoms:
- discomfort and pain in the upper abdomen after eating;
- a feeling of fullness in the projection of the stomach;
- belching with a putrid odor;
- vomiting food eaten;
- bloating;
- alternating constipation and diarrhea.
Protein Denaturation
Denaturation is the process of transforming the globular structure of a protein into a simple one. Initially, protein consists of amino acids connected in series. Next, disulfide bonds are formed between the chains, and it conforms (twists) into a compact structure - a globule. More often, these are tertiary and quaternary forms. This shape is explained by the need to correctly position the long chain.
For normal energy metabolism and obtaining important elements for structuring the protein structures of the human body. When exposed to acid, disulfide bonds are the first to break. The structure returns to the original serial circuit. It comes apart piece by piece, like a mosaic, and is included in processes (formation of RNA, muscle fibers, oxidation to produce energy).
Acidity as an indicator of stomach condition
The concentration of hydrochloric acid in the stomach not only shows how ready the organ is to eat food, but also regulates normal processes. Normally, the gastric mucosa is covered with secretion from the antral glands. This is a protective mucus. It can withstand a certain pH. The secretion is constantly produced to maintain the integrity of the mucosa and block the coagulation effect on the endothelium.
Normal stomach acidity
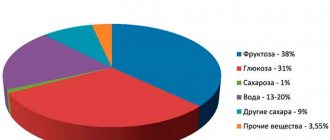
The acidity of the stomach constantly changes depending on the phase of digestion, the pH of the food consumed, and the presence of bacteria. Research has shown that the minimum component is 0.86; and the maximum is 8.3. In the antrum of the stomach, pH can vary from 1.3 to 7.4. These are indicators measured on mucous membranes. In the lumen of the organ, the acidity is higher, 1.2 - 2.0.
Free hydrochloric acid
The composition of gastric juice includes dissociated hydrochloric acid. It is written this way – H+ and Cl-. The study of its amount after a test meal is 20-40, 0.07-0.14% of the absolute concentration. This is an inactive form.
Bound hydrochloric acid
It is not a dissociated species associated with a specific protein. This is a compound that can interact with active substances and absorb essential nutrients. The reaction of the compound is less acidic than that of the bound acid.
Why doesn't stomach acid eat away at the stomach?
Digestion of food in the stomach is carried out by enzymes of gastric juice, which is produced by glands located in its wall. On an empty stomach, the stomach contains up to 50 ml of slightly acidic juice (pH 6.0 and above). When eating, juice is produced (up to 2.0-2.5 liters per day) with high acidity (pH 1.0-1.8).
One of the main components of gastric juice is hydrochloric acid; it performs several important functions: it participates in the digestion of proteins, regulation of the synthesis of stomach hormones (gastrin, secretin), evacuation of chyme into the duodenum, and also has a bactericidal effect.
The mucous-bicarbonate barrier (MBB) protects against the aggressive effects of gastric juice. The SBB lines the inside of the stomach and is a layer of thick mucus (1.0-1.5 mm) with bicarbonate ions located underneath (see figure). The secretion of bicarbonates parallels the secretion of hydrochloric acid: the more hydrochloric acid is produced, the more bicarbonates are produced, therefore, the higher the protective properties of SBB. Thus, the stomach is reliably protected from self-digestion! (ed.)
In some countries, people eat the stomach contents of other animals, for example, the Scottish dish haggis is a sheep stomach with meat from various organs and oatmeal, cooked on the stove. Our stomachs easily digest these stomachs. And our own stomachs remain unharmed after every acid shower after eating. Here's how it's explained. The stomach produces a lot of gastric juice per day - about 6 cups of sour brew.
Why doesn't stomach acid eat away at the stomach?
The protector here is mucus. Without it, stomach acid, which could dissolve even metal, would corrode our stomach. The walls of the stomach consist of many layers. The outer layer of the serous membrane is thin and dense; underneath there are three layers of muscles, which, by contracting, grind and mix food. The inner layer of the stomach wall, the epithelium, consists of many special cells. Some of them, parietal cells, secrete hydrochloric acid - HCl. Parietal and other cells also secrete enzymes that dissolve proteins.
Why don't the acids in the stomach eat away at it?
Digestion of food in the stomach is carried out by enzymes of gastric juice, which is produced by glands located in its wall. On an empty stomach, the stomach contains up to 50 ml of slightly acidic juice with a pH of 6.0 and higher. When eating, juice is produced up to 2.5 liters per day with a high acidity pH of 1.8. One of the main components of gastric juice is hydrochloric acid; it performs several important functions: it participates in the digestion of proteins, regulation of the synthesis of gastric hormones gastrin, secretin, evacuation of chyme into the duodenum, and also has a bactericidal effect. The mucous-bicarbonate barrier SBB protects from the aggressive effects of gastric juice. The SBB lines the lumen of the stomach from the inside and is a 1.5 mm layer of thick mucus with bicarbonate ions located underneath it (see figure). The secretion of bicarbonates runs parallel to the secretion of hydrochloric acid: the more hydrochloric acid is produced, the more bicarbonates are produced, therefore, the higher the protective properties of SBB. Thus, the stomach is reliably protected from self-digestion! Because the inside of the stomach is covered with a multi-layered mucous membrane, which does not allow it to be corroded.
Personal experience. 6 years ago I was diagnosed with the above disease. I tried to stick to a special diet, but somehow it still didn’t help, the disease progressed. As a result, I was taken to the ambulance before the New Year with acute pain. I complained about this to a friend and he advised me of an effective natural remedy. And after about two weeks I felt much better. Then I also bought myself a recovery course. In short, in a couple of months I completely cured this disease, the tests were normal, but the attending physician was shocked. If you are interested in learning about the product, be sure to check out the interesting article at this link.
Source
Methods for studying the acidity of gastric juice
To check, intragastric pH-metry, or fractional sounding, is used. To study acidity, phenolphthalein, dimethylaminoazobenzene and alizarine sulfonic acid indicators are used. Phenolphthalein, when the pH shifts to the alkaline side, acquires a characteristic pink or crimson color.
Dimethylaminoazobenzene stripes turn red if the environment is acidic and free hydrogen chloride predominates. An increased concentration of proteinized hydrochloric acid is indicated by orange color.
What happens with low acidity
Hyposecretion of gastric juice is much less common. Do not assume that this condition is better (based on information obtained from television advertising). On the contrary, gastric hypofunction is much more dangerous.
Some people do not know how much acid a person should produce, and they believe that the less it is, the better, because then “there will be no heartburn.” The mechanism of the stomach is such that for its normal function, its secretion must have an acidic reaction. If little acid is produced, gastric activity decreases, and many disease-causing organisms can enter the body.
What does a person feel with low acidity? There is no need to think that this changes the color of the gastric discharge. It has reduced enzymatic properties, which contributes to the appearance of the following symptoms:
- a sharp drop in appetite;
- belching with an unpleasant odor of spoiled eggs;
- bad odor coming from the mouth and not going away after brushing your teeth;
- constipation;
- signs of intestinal upset;
- nausea, worse after eating;
- the presence of helminths in the stomach or intestines (they are not neutralized by acid);
- flatulence.
Interesting! Chemical composition of gastric juice in normal and pathological conditions
The danger of this condition is as follows:
- due to a decrease in the intensity of digestive processes, a large amount of food accumulates in the body
- amount of decomposition products;
- decreased absorption leads to anemia, hair loss, etc.;
- the development of autoimmune pathologies and even cancer;
- the appearance of allergic reactions even to once familiar products;
- due to a decrease in the effect of gastric juice on proteins, the patient may develop protein starvation;
- decrease in blood pressure.
To treat this condition, it is necessary to choose a therapy that gives the juice normal acidity. Sometimes the patient needs to consume hydrochloric acid preparations.
Acid-dependent diseases of the gastrointestinal tract
A healthy body has strong protection and homeostasis, thanks to which normal digestive functions are performed. The first and most famous disease associated with changes in acidity is gastritis. The mucous secretion cannot properly protect the mucosa from the effects of pathogens. This occurs due to:
- disruption of antral cell secretion;
- changes in the composition of mucus;
- distortion of normal HCl release;
- constant intake of acidic foods.
Gastric ulcer, one of the complications of gastritis, the appearance of a defect in the gastric mucosa. It is dangerous due to malignancy, perforation, and penitration. This disease directly depends on the amount of Helicobacter pylori and its effect on the gastric mucosa. By parasitizing the endothelium, it destroys the mucous layer that protects the membrane. As a result, the acid causes necrosis and ulceration.
Secretion of gastric juice
The secretion of gastric juice is determined in the first, complex reflex phase of secretion by the sight, smell and taste of food; in the second, neuro-humoral phase - chemical and mechanical irritations of the gastric mucosa. A person secretes up to 2 liters of gastric juice per day. The quantity, composition and properties of gastric juice vary depending on the nature of the food, as well as in diseases of the stomach, intestines, and liver.
The actual process of secreting gastric juice is activated when peptides are in the stomach and the hormone gastrin begins to enter the blood, which induces the gastric glands to secrete gastric juice.
Secretion phases
The phases of gastric secretion are phases of activation of the formation of gastric juice, caused by various nervous humoral regulatory mechanisms. In the cerebral (complex reflex) phase, gastric secretion is activated by sight, smell, preparation of food for consumption through receptors of vision, hearing, (conditioned reflex excitations) and when food enters the oral cavity and thereby excites the receptors of the mouth, tongue, palate, pharynx ( insanely reflex secretion gastric (neuro-humoral) phase occurs with mechanical and chemical irritation of the receptors of the gastric mucosa with food, as well as under the influence of humoral factors (histamine, gastrin, etc.); the intestinal phase occurs when gastric contents enter the intestine, causing the release of endocrinocytes the intestinal mucosa of hormones, in particular enterogstrin (the main powerful humoral factor), which stimulate the secretion of gastric juice through the blood.
Signs, causes and consequences of increased acidity
Regulating acidity is an independent process. The body reacts to any change in a positive or negative direction by activating its defense systems. Increased acidity occurs when it cannot accurately control secretions.
The first symptoms are heartburn, sour belching, and hungry abdominal pain. They occur as a result of gastritis, poor diet, peptic ulcers, large amounts of Helicobacter pylori, and alcoholism. Increased acidity can significantly reduce a person's quality of life.
Signs, causes and consequences of low pH
A decrease in acidity is caused by systematic overeating, fasting, poor diet, stress, sympathoactive nervous regulation, lack of vitamins, in particular PP and B1, and lack of zinc. Violation of concentration leads to a distortion of the optimal environment, opportunistic microflora multiplies, and the body becomes infected.
Along with this, insufficient activation of enzymes causes abnormal digestion. The disease causes iron deficiency anemia, a lack of B12, C, A, and beneficial elements.
How can you reduce or increase the concentration of hydrochloric acid in the stomach?
The normal digestive ability of the gastrointestinal tract lies in the coordinated work of the gastrointestinal tract and other body systems.
Why is hydrochloric acid needed in the stomach?
A sufficient level of secretion ensures the process of food processing and prepares chyme for further absorption of nutrients in the intestine.
Inflammatory diseases of the upper digestive tract lead to disruption of the synthesis of gastric juice. Violation of secretion concentration forms organic and functional changes in the organ. Its lack slows down the breakdown of food components and increases the likelihood of the proliferation of pathogenic bacteria. When there is a lot of hydrochloric acid, it leads to severe inflammation and destruction of the epithelium.
The medications presented in Table 2 help raise or lower stomach acidity.
Table 2. Main groups of drugs affecting the synthesis of hydrochloric acid
| Reduce pH | Increase pH |
| Proton pump blockers | Natural gastric juice |
| H2-histamine blockers | Stimulators of gastric juice synthesis |
| Antacids |
Hydrochloric acid is an important functional component of digestion. An imbalance in the process of production and neutralization of secretions leads to negative changes requiring treatment.
pH normalization methods
There are two types of effects: neutralizing the pH and changing the rate and amount of HCl release. Reducing pH with antacids, “ Pechaevskie ”, “ Rennie ”, “ Phospholugel ”. A solution of baking soda can be used in everyday life, but when the acid is neutralized, CO2 is formed, which bloats the stomach and can lead to pain and severe belching.
To normalize the endocrine level, H2-histamine receptor blockers and proton pump inhibitors are used: Omeprazole , Dexlansoprazole , Esomeprazole .