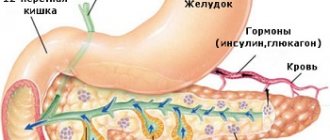
Pancreatic tissue is represented by two types of cellular formations: the acinus, which produces enzymes and is involved in digestive function, and the islet of Langerhans, whose main function is to synthesize hormones.
There are few islets in the gland itself: they make up 1-2% of the total mass of the organ. The cells of the islets of Langerhans differ in structure and function. There are 5 types of them. They secrete active substances that regulate carbohydrate metabolism, digestion, and can participate in the response to stress reactions.
Hormonal activity of the islets of Langerhans
The small size of islet accumulations, as well as the small area they occupy in the pancreas, is an indisputable fact. However, the importance of this structure for the entire organism as a whole is very great, because it is in it that hormones are formed that take part in the metabolic process. This includes not only insulin, but also somatostatin, glucagon, and pancreatic polypeptide. Let's consider their main purpose.
- Insulin is necessary to regulate carbohydrate balance, maintain adequate blood glucose levels, transport potassium, fats, glucose and amino acids into cells. In addition, this hormone is involved in the formation of glycogen, it affects the synthesis of fats and proteins, and also increases the permeability of the plasma membrane.
- The hormone glucagon has a whole list of functions, which:
- Promotes the breakdown of glycogen, due to which glucose is released;
- Triggers the breakdown of lipids: when the level of lipase increases in fat cells, lipid breakdown products begin to enter the blood, acting as sources of energy;
- Provides rapid removal of sodium from the body, thereby improving the functioning of blood vessels and the heart;
- Increases calcium concentration inside cells;
- Improves blood flow to the kidneys;
- Activates the formation of glucose from substances that are not components of the carbohydrate group;
- Increases blood pressure;
- Promotes the restoration of liver cells;
- It has an antispasmodic effect at particularly high concentrations.
- The delta cell hormone somatostatin controls the production of digestive enzymes as well as other hormones. Due to its effect, there is a decrease in the production of insulin and glucagon.
- Pancreatic polypeptide - it is produced by PP cells, and despite the fact that there are very few of them in islet accumulations, the significance of this substance is very important: the polypeptide takes an active part in controlling the secretion of the stomach and liver. It is known that with insufficient amounts of this hormone, various pathological processes develop.
Mechanism of hormonal action and function
The non-hormonal substance C-peptide is a fragment of the insulin molecule. During its synthesis, it breaks away from its native cell and enters the circulatory system. Its volume is equivalent to the volume of insulin.
Since there are no chemical reactions in it, it is considered the most accurate indicator of the presence of insulin in the blood. In modern medicine, it is used to diagnose insulin-dependent and non-insulin-dependent diabetes, as well as neoplasms and liver pathologies. The pancreas has tasks for all hormones produced within its confines:
Glucagon
The task of which is to monitor the decline in glucose and its increase to normal in the blood. Mechanism of action:
- glucagon stimulates an increase in the availability of glucose in the blood system as a result of accumulation in the liver and muscles;
- glucagon also stimulates the breakdown of lipids based in adipose tissue, resulting in a new source of energy;
- gluconeogenesis, which leads to the creation of glucose from non-carbohydrate components. It is needed for many areas that need glucose. Here we can name red blood cells and tissues of the nervous system.
Insulin
Which is the key hormone of the pancreas. Its main purpose is to lower blood glucose. Demotion uses different mechanisms of action:
- to bring glucose molecules into cells, insulin activates membrane receptors;
- transfer of excess glucose into the liver in the form of glycogen. The process is supported by insulin;
- inhibition of gluconeogenesis, i.e., does not allow the breakdown of non-carbohydrate sources of glucose from elements;
- insulin promotes the transport of beneficial elements magnesium, potassium, amino acids, and phosphates into the cell;
- increasing protein biosynthesis, suppressing its hydrolysis, which contributes to the absence of protein deficiency in the body and the development of full immunity;
- insulin, on the one hand, synthesizes fatty acids for further activation of fat reserves, on the other hand, prohibits their penetration into the blood. Thus, the amount of bad cholesterol is reduced, which is the prevention of atherosclerosis.
Samotostatin
The pancreas has its own influential brake in the form of somatostatin on its other enzymes and hormones. This hormone is produced in the cells of the small intestine, hypothalamus, and nervous system. It maintains digestive balance by regulating this process through the following actions:
- inhibits the movement of crushed food from the stomach into the intestinal tract;
- slows down the production of stomach acid and gastrin;
- inhibits the dynamics of pancreatic enzymes;
- reduces blood circulation in the retroperitoneal space;
- suppresses the absorption of carbohydrates from the peptic tract;
- lowers glucagon levels.
Polypeptide
The pancreas secretes pancreatic polypeptide among PP cells. According to the mechanism of action, it serves as a cholecystokin antagonist. By suppressing the secretory activity of the gland, it stimulates the production of gastric juice. The hormone is young in discovery and is under study. What is already known:
- restraining the surge in the release of bilirubin, bile, trypsin into the blood;
- the ability to relax the smooth muscles of the gallbladder;
- inhibition of the production of enzymes involved in digestion.
The main task of the pancreatic polypeptide appears to be the saving of digestive enzymes, as well as bile, which is saved until the next meal.
Gastrin
This is a hormone of 2 organs: the stomach and pancreas. In terms of its volume, it has less iron. It controls the functioning of those hormones that are involved in the process of digestion of food. Failures in its production affect the functioning of the gastrointestinal tract. To rule out stomach ulcers, a gastrin test is performed. Gastrin has 3 types:
- large, having 34 amino acids at its disposal;
- small, consisting of 17 amino acids;
- gastrin micro, has 14 amino acids in its set.
How are islands arranged and what is their purpose?
The main task of the islets of Langerhans is to maintain carbohydrate balance, as well as to control the activity of all endocrine organs. These clusters are very well supplied with blood, and their innervation occurs due to the vagus and sympathetic nerves.
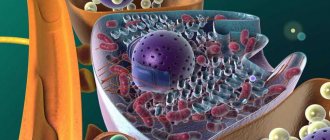
The structure of the islands is quite complex; their cells are arranged in a chaotic order, like a mosaic. Each of the clusters is an independent perfect formation, consisting of lobules surrounded by connective tissues and having blood capillaries passing through the cells. Beta cells are located in the center of the clusters, alpha and delta cells make up the periphery. By interacting with each other, cells trigger a feedback mechanism, characterized by the influence of some cells on others located nearby:
- Alpha cells produce glucagon, which in turn has a specific effect on d cells;
- Somatostatin, produced by d-cells, inhibits the activity of alpha and beta cells;
- It suppresses alpha cells and insulin, but at the same time, it activates beta cells.
When any disruption occurs in the functioning of the immune system, special immune bodies arise, leading to dysfunction of beta cells, resulting in the development of a pathology such as diabetes mellitus (DM).
Histological structure
Each island is an independently functioning element. Together they form a complex archipelago, which is made up of individual cells and larger formations. Their sizes vary significantly - from one endocrine cell to a mature, large islet (>100 µm).
In pancreatic groups, a hierarchy of cell arrangement is built, there are 5 types of them, all perform their role. Each island is surrounded by connective tissue and has lobules where capillaries are located.
In the center there are groups of beta cells, along the edges of the formations there are alpha and delta cells. The larger the islet, the more peripheral cells it contains.
The islets do not have ducts; the hormones produced are excreted through the capillary system.
A little anatomy
The pancreatic tissue contains not only acini, but also islets of Langerhans. The cells of these formations do not produce enzymes. Their main function is to produce hormones.
These endocrine cells were first discovered in the 19th century. The scientist after whom these formations are named was still a student at that time.
There are not so many islands in the iron itself. Among the entire mass of the organ, Langerhans' zones account for 1-2%. However, their role is great. The cells of the endocrine part of the gland produce 5 types of hormones that regulate digestion, carbohydrate metabolism, and stress response. With the pathology of these active zones, one of the common diseases of the 21st century develops - diabetes mellitus. In addition, the pathology of these cells causes Zollinger-Ellison syndrome, insulinoma, glucoganoma and other rare diseases.
Today it is known that pancreatic islets have 5 types of cells. Let's talk more about their function below.
Alpha cells
These cells make up 15-20% of the total islet cells. It is known that humans have more alpha cells than animals. These zones secrete hormones responsible for the fight and flight response. Glucagon, which is formed here, sharply increases glucose levels, enhances the work of skeletal muscles, and speeds up the heart. Glucagon also stimulates the production of adrenaline.
Glucagon is designed for a short duration of action. It is quickly destroyed in the blood. The second significant function of this substance is insulin antagonism. Glucagon is released when there is a sharp decrease in blood glucose. Such hormones are administered in hospitals to patients with hypoglycemic conditions and coma.
Beta cells
These areas of parenchymal tissue secrete insulin. They are the most numerous (about 80% of cells). They can be found not only in the islets; there are single zones of insulin secretion in the acini and ducts.
The function of insulin is to lower glucose concentrations. Hormones make cell membranes permeable. Thanks to this, the sugar molecule quickly gets inside. Further, they activate a chain of reactions that produce energy from glucose (glycolysis) and store it in reserve (in the form of glycogen), and form fats and proteins from it. If insulin is not secreted by cells, type 1 diabetes develops. If the hormone does not act on the tissue, type 2 diabetes mellitus is formed.
Insulin production is a complex process. Its level can be increased by carbohydrates from food and amino acids (especially leucine and arginine). Insulin increases with an increase in calcium, potassium and some hormonally active substances (ACTH, estrogen and others).
C-peptide is also formed in the beta zones. What it is? This word refers to one of the metabolites that is formed during the synthesis of insulin
Recently, this molecule has acquired important clinical significance. When an insulin molecule is formed, one molecule of C-peptide is formed
But the latter has a longer decay life in the body (insulin lasts no more than 4 minutes, and C-peptide about 20). C-peptide decreases in type 1 diabetes mellitus (little insulin is initially produced), and increases in type 2 diabetes (there is a lot of insulin, but the tissues do not respond to it), insulinoma.
Delta cells
These are zones of pancreatic tissue of Langerhans cells that secrete somatostatin. The hormone inhibits the activity of enzyme secretion. The substance also slows down other organs of the endocrine system (hypothalamus and pituitary gland). The clinic uses a synthetic analogue or Sandostatin. The drug is actively administered during attacks of pancreatitis and pancreas operations.
Hormonal activity
The hormonal role of the pancreas is great.
Active substances synthesized in small islets are delivered to the organs by the bloodstream and regulate the metabolism of carbohydrates:
- The main job of insulin is to minimize blood sugar levels. It increases the absorption of glucose by cell membranes, accelerates its oxidation and helps to store it in the form of glycogen. Violation of hormone synthesis leads to the development of type 1 diabetes. In this case, blood tests show the presence of antibodies to beta cells. Type 2 diabetes develops when tissue sensitivity to insulin decreases.
- Glucagon performs the opposite function - it increases sugar levels, regulates glucose production in the liver, and accelerates the breakdown of lipids. The two hormones, complementing each other’s action, harmonize the content of glucose, a substance that ensures the vital activity of the body at the cellular level.
- Somatostatin slows down the action of many hormones. In this case, there is a decrease in the rate of absorption of sugar from food, a decrease in the synthesis of digestive enzymes, and a decrease in the amount of glucagon.
- Pancreatic polypeptide reduces the number of enzymes and slows down the release of bile and bilirubin. It is believed that it stops the consumption of digestive enzymes, preserving them until the next meal.
- Ghrelin is considered a hunger or satiety hormone. Its production signals the body about the feeling of hunger.
The amount of hormones produced depends on the glucose obtained from food and the rate of its oxidation. As its amount increases, insulin production increases. Synthesis starts at a concentration of 5.5 mmol/l in blood plasma.
Not only food intake can trigger insulin production. In a healthy person, the maximum concentration is observed during periods of severe physical exertion and stress.
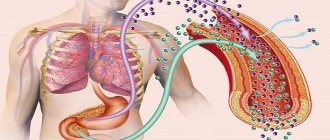
The endocrine part of the pancreas produces hormones that have a decisive influence on the entire body. Pathological changes in the OB can disrupt the functioning of all organs.
Video about the tasks of insulin in the human body:
Islets of Langerhans of the pancreas
Groups of such cells were discovered back in 1869 by the scientist Paul Langerhans, after whom they were named. Islet cells are concentrated predominantly in the tail of the pancreas and account for 2% of the organ's mass. In total, there are about 1 million islets in the parenchyma.
It was revealed that in newborns the islets occupy 6% of the total mass of the organ. As the body ages, the proportion of structures with endocrine activity decreases. By the age of 50, only 1-2% remain. During the day, the islets of Langerhans secrete 2 mg of insulin.
What cells are islets made of?
The islets of Langerhans contain different, morphologically and functionally, cells.
The endocrine segment of the pancreas includes:
- Alpha cells - produce glucagon, which is an insulin antagonist and ensures an increase in plasma glucose levels. They occupy 20% of the mass of the remaining cells.
- Beta cells - synthesize insulin and ameline. They make up 80% of the island's mass.
- Delta cells - provide the production of somatostatin, which can inhibit the secretion of other glands. These cells make up from 3 to 10% of the total mass.
- PP cells – produce pancreatic polypeptide. It is responsible for increasing gastric secretion and suppressing pancreatic function.
- Epsilon cells secrete ghrelin, which is responsible for the feeling of hunger.
Why are islands needed and how are they arranged?
The islets of Langerhans are responsible for maintaining the balance of carbohydrates in the body and the functioning of other endocrine organs. They have an abundant blood supply and are innervated by the vagus and sympathetic nerves. Among the islets are neuroinsular complexes. Ontogenetically, islet cells are formed from epithelial tissue.
The islet has a complex structure and each of them is a full-fledged functionally active formation.
Its structure promotes the exchange of biologically active substances between other glands for the simultaneous secretion of insulin. The cells of the islets are arranged in the form of a mosaic, that is, they are mixed together.
The exocrine structure of the pancreas can be represented by clusters of several cells and large islets.
It is known that a mature islet in the parenchyma has an ordered organization. It is surrounded by connective tissue, has lobules, and blood capillaries run inside. The center of the lobule is filled with beta cells, and alpha and delta cells are located on the periphery. We can say that the structure of the island is directly related to its size.
What is the endocrine function of islets and why are antibodies formed against them? When islet cells interact, a feedback mechanism is formed. Cells influence nearby:
- Insulin has an activating effect on beta cells and inhibits alpha cells.
- Glucagon activates alpha cells, which in turn influence delta cells.
- Somatostatin inhibits the functioning of alpha and beta cells.
When immune mechanisms against beta cells are disrupted, antibodies are formed that destroy them and lead to the development of diabetes mellitus.
Why is islets transplanted?
Islet transplantation serves as a worthy alternative to pancreas transplantation or installation of an artificial organ. This intervention gives patients with diabetes a chance to restore the structure of beta cells.
Clinical studies were conducted in which islet cells from donors were transplanted into patients with type 1 diabetes. As a result of the tests, it was revealed that such an intervention leads to the restoration of regulation of carbohydrate levels.
Patients with diabetes undergo powerful immunosuppressive therapy to prevent rejection of donor tissue.
An alternative source of material for islet repair is stem cells. They may be relevant since donor cell reserves are limited.
Regenerative medicine is developing rapidly, offering new treatments in many areas
It is important to restore the immune system's tolerance, since the new transplanted cells will also be destroyed after a certain period of time.
Xenotransplantation—transplantation of a pancreas from a pig—has promise. Before the discovery of insulin, extracts from pork pancreas were used to treat diabetes.
It is known that human and pork insulin differ in only one amino acid.
The study of the structure and function of the islets of Langerhans has great prospects, since diabetes mellitus develops due to damage to their structure.
Author : Nikulina Natalya Viktorovna, especially for the site Moizhivot.ru
about the pancreas
Source:
What are the islets of Langerhans?
The islets of Langerhans (OL) are polyhormonal microorgans consisting of endocrine cells located throughout the entire length of the pancreatic parenchyma, which performs exocrine functions. Their bulk is localized in the tail part. The size of the islets of Langerhans is 0.1-0.2 mm, their total number in the human pancreas ranges from 200 thousand to 1.8 million.
The cells form separate groups, between which capillary vessels pass. They are delimited from the glandular epithelium of the acini by connective tissue and nerve cell fibers running there. These elements of the nervous system and the cells of the islet form the neuroinsular complex.
The structural elements of the islets—hormones—perform intrasecretory functions: they regulate carbohydrate and lipid metabolism, digestive processes, and metabolism. The child's gland contains 6% of these hormonal formations of the total area of the organ. In an adult, this part of the pancreas is significantly reduced and accounts for 2% of the surface of the gland.
History of discovery
Clusters of cells, different in appearance and morphological structure from the main tissue of the gland and located in small groups mainly in the tail of the pancreas, were first discovered in 1869 by the German pathologist Paul Langerhans (1849-1888).
In 1881, the outstanding Russian scientist, pathophysiologist K.P. Ulezko-Stroganova (1858-1943) carried out fundamental physiological and histological work on the study of the pancreas. The results were published in the journal “Doctor”, 1883, No. 21 - article “On the structure of the pancreas under conditions of its rest and activity.” In it, for the first time at that time, she expressed a hypothesis about the endocrine function of individual pancreatic formations.
Based on her work in 1889-1892. in Germany, O. Minkovsky and D. Mehring found that when the pancreas is removed, diabetes mellitus develops, which can be eliminated by transplanting part of a healthy pancreas under the skin of the operated animal.
Domestic scientist L.V. Sobolev (1876-1921) was one of the first, based on research work, to show the importance of the islets discovered by Langerhans and named after him in the production of a substance related to the occurrence of diabetes mellitus.
Subsequently, thanks to a large number of studies conducted by physiologists in Russia and other countries, new scientific data on the endocrine function of the pancreas were discovered. In 1990, the first transplantation of islets of Langerhans into humans was performed.
How are islands arranged and what are they for?
The main function performed by the islets of Langerhans is to maintain the correct level of carbohydrates in the body and control other endocrine organs.
The islets are innervated by sympathetic and vagus nerves and are abundantly supplied with blood. The islets of Langerhans in the pancreas have a complex structure. In fact, each of them is an active, full-fledged functional formation. The structure of the islet ensures the exchange between biologically active substances of the parenchyma and other glands. This is necessary for the smooth secretion of insulin.
The cells of the islets are mixed among themselves, that is, they are arranged in the form of a mosaic. A mature islet in the pancreas has the correct organization. The islet consists of lobules that are surrounded by connective tissue; blood capillaries pass inside the cells.
In the center of the lobules there are beta cells, while in the peripheral part there are alpha and delta cells. Therefore, the structure of the islets of Langerhans depends entirely on their size.
Why are antibodies formed against islets? What is their endocrine function? It turns out that when islet cells interact, a feedback mechanism develops, and then these cells influence other cells located nearby.
- Insulin activates the function of beta cells and inhibits alpha cells.
- Alpha cells are activated by glucagon, which acts on delta cells.
- The work of alpha and beta cells is inhibited by somatostatin.
Important! When immune mechanisms fail, immune bodies directed against beta cells are formed. Cells are destroyed and lead to a terrible disease called diabetes mellitus.
Hormones and their functions
The endocrine system is responsible for hormonal balance. It consists of various glands and organs, the most important of which are considered:
- thyroid gland;
- pancreas;
- adrenal glands
Adrenal hormones and their functions have a direct connection with the central nervous system and are divided into 2 groups: the cortex and the medulla.
In addition, the secretions of the glands regulate the functioning of the digestive system (for example, they allow a person to cope with the feeling of hunger) and affect metabolic processes.
To understand what hormones the adrenal glands produce, you need to consider each of their subgroups, namely those parts where the secretion production itself occurs:
- medulla;
- cortical component;
- secret of the reticular zone.
Diseases of the cells of the islets of Langerhans
The cellular system of the islets of Langerhans in the gland can be destroyed.
This occurs during the following pathological processes: autoimmune reactions, oncology, pancreatic necrosis, acute exotoxicosis, endotoxicosis, systemic diseases.
Elderly people are also susceptible to the disease. Illnesses occur in the presence of serious growth of destruction.
This occurs when cells are susceptible to tumor-like phenomena. The neoplasms themselves are hormone-producing, and therefore are accompanied by signs of failure of the hyperfunction of the pancreatic organ.
There are several types of pathologies associated with the destruction of the gland. The critical rate is if the loss is more than 80 percent of the islets of Langerhans.
With the destruction of the pancreas, the production of insulin is impaired, and therefore the hormone is not enough to process the sugar that enters the body.
Due to this failure, the development of diabetes is observed. It is worth noting that diabetes mellitus of the first and second degree must be understood as two different pathologies.
In the second case, the increase in sugar levels will be related to the fact that the cells are not sensitive to insulin. As for the functioning of Langerhans zones, they operate as before.
The destruction of hormone-forming structures provokes the development of diabetes mellitus. This phenomenon is characterized by a number of signs of failure.
These include the appearance of dry mouth and constant thirst. In this case, there may be attacks of nausea or increased nervous excitability.
A person may experience insomnia and a sharp loss of body weight, despite eating heavily.
If the sugar level in the body increases, it is possible that an unpleasant acetone smell will appear in the mouth. Possibly impaired consciousness and a hyperglycemic state of coma.
From the above information, it is worth concluding that pancreatic cells are capable of producing a number of hormones needed by the body.
Without them, the full functioning of the body will be disrupted. These hormones carry out carbohydrate metabolism and a number of anabolic processes.
Destruction of the zones will lead to the development of complications associated with the need for hormonal therapy in the future.
To avoid the need for such events to develop, it is recommended to adhere to special recommendations from specialists.
Basically, they boil down to the fact that you should not consume alcoholic beverages in large doses, it is important to promptly treat infectious pathologies and autoimmune disorders in the body, and visit a doctor at the first signs of an illness associated with damage to the pancreas and other organs included in the gastrointestinal tract. .
Cortical hormones
The cortex of the glands plays an important role in the production of secretions of the corticosteroid group.
Considering that the structure of the adrenal cortex, like hormones, is not simple, its 3 tiers can have different effects on the body:
- zona glomerulosa;
- fascicular;
- mesh.
The following adrenal hormones are produced in the glomerular region:
- Aldosterone - takes part in the regulation of water-salt balance and hemodynamics, increases blood pressure, and increases the amount of circulating blood.
- Corticosterone – regulates water-salt balance.
- Deoxycorticosterone – increases the body's endurance.
The cortical layer of the adrenal glands of the zona fasciculata produces the following active components:
- Cortisol (allows the human body to accumulate energy, regulates carbohydrate metabolism).
- Corticosterone.
What is a transplant and why is it needed?
A worthy alternative to gland parenchyma transplantation is islet transplantation. In this case, installation of an artificial organ will not be required. The transplant gives diabetics a chance to restore their beta cell structure and does not require a full pancreas transplant.
Based on clinical studies, it has been proven that in patients with type 1 diabetes mellitus who were transplanted with donor islet cells, the regulation of carbohydrate levels is completely restored. To prevent rejection of donor tissue, such patients were given powerful immunosuppressive therapy.
There is another material for restoring islets - stem cells. Since the reserves of donor cells are not unlimited, this alternative is very relevant.
It is very important for the body to restore the sensitivity of the immune system, otherwise the newly transplanted cells will be rejected or destroyed after some time. . Today, regeneration therapy is developing rapidly, offering new techniques in all areas
Xenotransplantation, the transplantation of a pig pancreas into a person, is also promising.
Today, regeneration therapy is developing rapidly, offering new techniques in all areas. Xenotransplantation, the transplantation of a pig pancreas into a person, is also promising.
Porcine parenchyma extracts were used to treat diabetes mellitus even before the discovery of insulin. It turns out that human and pork glands differ in only one amino acid.
Since diabetes mellitus develops as a result of damage to the islets of Langerhans, their study has great prospects for effective treatment of the disease.
What are the advantages and disadvantages of pancreatic islet allotransplantation?
Benefits of islet allotransplantation include improved blood glucose control, reduced or eliminated need for insulin injections for diabetes, and prevention of hypoglycemia. An alternative to pancreatic islet transplantation is a whole pancreas transplant, which is most often performed in conjunction with a kidney transplant.
The advantages of transplanting the entire pancreas are less dependence on insulin and longer functioning of the organ. The main disadvantage of a pancreas transplant is that it is a very complex operation with a high risk of complications and even death.
Pancreatic islet allotransplantation may also help avoid unknowing hypoglycemia. Scientific studies have shown that even partially functioning islets after transplantation can prevent this dangerous condition.
Pancreas
The organ belongs to the endocrine and digestive systems. It produces enzymes that break down food that enters the body. Also hormones that regulate carbohydrate and fat metabolism. The pancreas consists of lobules, each of which produces substances necessary for the body - enzymes. It is shaped like an elongated comma. Weighs from 80 to 90 g. The organ is located behind the stomach.
The gland consists of:
- heads;
- necks;
- bodies (triangular shape);
- tail (pear-shaped).
Important. The organ is equipped with blood vessels and excretory ducts
A channel passes through the entire gland, through which the produced pancreatic juice is discharged into the duodenum.
Enzymes produced by the pancreas include:
- amylase;
- lactase;
- trypsin;
- lipase;
- invertase.
Special cells, insulocytes, carry out the endocrine mission of the pancreas. They produce the following hormones:
- Gastrin.
- Insulin.
- C-peptide.
- Thiroliberin.
- Glucagon.
- Somatostatin.
Important. Hormones are involved in the body's carbohydrate metabolism.
Malignant tumors of the gland
The formation of a head in the pancreas of a malignant type is a serious problem that is very difficult to cure. At the same time, the level of bile patency in the ducts, as well as in the duodenum, drops sharply. A tumor can grow into the stomach if it initially affects the body of the gland. Tail tumors often spread to the vascular system of the spleen, and the tumor begins to cover the entire pancreas.
Symptoms
At later stages of development of malignant neoplasms, signs appear, which, however, can be a manifestation of other diseases:
- regular pain in the stomach, becoming more pronounced at night;
- loss of appetite, lack of cravings for meat dishes, coffee or fatty foods;
- rapid reduction in body weight;
- insomnia and weakness;
- enlarged gallbladder;
- manifestations of thrombotic disorders of peripheral veins;
- jaundice;
- digestive problems, heaviness in the stomach;
- bleeding, manifested by blackening of stool;
- thirst and dry mouth;
- skin itching;
- accumulation of fluid in the abdominal cavity.
Diagnosis and prognosis
To make a diagnosis, the following instrumental diagnostic methods are used:
- Ultrasound – volumetric neoplasms larger than 20 mm in size are determined with a high degree of accuracy;
- CT scan, which evaluates the location of the tumor, its dimensions and shape, as well as the presence of metastases and the risk of germination;
- MRI helps detect small tumors and assess the spread of formations;
- Positron emission tomography - plays an important role in diagnosing cancer-type tumors;
- Irrigographic and x-ray examination of the stomach. X-rays provide information about the degree of deformation of organs and are performed using contrast.
- Performing gastroscopy;
- Taking a biopsy using fibrogastroduodenoscopy and oral cholangiopancreatography.
Malignant changes detected at early or late stages are difficult to treat, and the prognosis is usually unfavorable. The pancreas responds poorly to chemotherapy courses, is not subject to surgical manipulation, and the tumor quickly spreads to other organs.
Functional Features
The main hormone produced by the islets of Langerhans is insulin. But it should be noted that Langerhans' zones produce certain hormones with each cell.
For example, alpha cells produce glucagon, beta cells produce insulin, and delta cells produce somatostatin.
PP cells - pancreatic polypeptide, epsilon - ghrelin. All hormones affect carbohydrate metabolism, lowering or increasing blood glucose levels.
Therefore, it must be said that pancreatic cells perform the main function associated with maintaining an adequate concentration of stored and free carbohydrates in the body.
In addition, substances produced by the gland influence the formation of fat or muscle mass.
They are also responsible for the functionality of certain brain structures associated with the suppression of the secretion of the hypothalamus and pituitary gland.
From this it is worth concluding that the main functions of the islets of Langerhans will be to maintain the correct level of carbohydrates in the body and control over other organs of the endocrine system.
They are innervated by the vagus and sympathetic nerves, which are abundantly supplied with blood flow.
Types of cells
Different groups of cells produce their own type of hormone, regulating digestion, lipid and carbohydrate metabolism.
- Alpha cells . This group of OBs is located along the edge of the islets; their volume makes up 15-20% of the total size. They synthesize glucagon, a hormone that regulates the amount of glucose in the blood.
- Beta cells . They are grouped in the center of the islets and make up most of their volume, 60-80%. They synthesize insulin, about 2 mg per day.
- Delta cells . They are responsible for the production of somatostatin, they range from 3 to 10%.
- Epsilon cells . The amount of the total mass is not more than 1%. Their product is ghrelin.
- PP cells . The hormone pancreatic polypeptide is produced by this part of the OB. They make up up to 5% of the islands.
Over the course of life, the proportion of the endocrine component of the pancreas decreases - from 6% in the first months of life to 1-2% by the age of 50.
Terms and Definitions
- Langerhans cell histiocytosis is a tumor of myeloid nature, the morphological substrate of which is pathological Langerhans cells, phenotypically similar to epidermal Langerhans cells
- Inactive disease (ID) is a disease status in which all reversible lesions have undergone reverse development
- Active disease (AD) is a disease status in which the original lesions persist or new lesions are detected
- Reactivation of the disease - the appearance of new lesions after achieving NAZ status
- Risk organs are organs (liver, spleen, bone marrow), the involvement of which in the pathological process is associated with a poor prognosis of the disease.
- Permanent complications (PC) are irreversible changes in the structure and/or function of organs as a result of lesions in LCH.
- Criteria for response to therapy
Islet cell transplantation
Transplantation of OB cells is an alternative to transplantation of the pancreas or part thereof, as well as the installation of an artificial organ. This is due to the high sensitivity and tenderness of the pancreas tissue to any influence: it is easily injured and has difficulty restoring its functions.
Islet transplantation today makes it possible to treat type I diabetes mellitus in cases where insulin replacement therapy has reached its limits and becomes ineffective. The method was first used by Canadian specialists and consists of introducing healthy endocrine donor cells into the portal vein of the liver into the patient using a catheter. It is aimed at making the remaining own beta cells work.
Due to the functioning of the transplanted cells, the amount of insulin necessary to maintain normal blood glucose levels is gradually synthesized. The effect comes quickly: with a successful operation, after two weeks the patient’s condition begins to improve, replacement therapy wears off, and the pancreas begins to independently synthesize insulin.
The danger of the operation lies in the rejection of the transplanted cells. We use cadaveric materials that are carefully selected according to all parameters of tissue compatibility. Since there are about 20 such criteria, antibodies present in the body can lead to the destruction of pancreatic tissue. Therefore, proper drug treatment aimed at reducing immune reactions plays an important role. The drugs are selected in such a way as to selectively block some of them that affect the production of antibodies to the cells of the transplanted islets of Langerhans. This allows you to minimize the risk to the pancreas.
In practice, pancreatic cell transplantation for type I diabetes mellitus shows good results: there have been no recorded deaths after such an operation. A certain number of patients significantly reduced their insulin dose, and some of the operated patients no longer needed it. Other impaired functions of the organ were also restored, and health improved. A significant part has returned to a normal lifestyle, which allows us to hope for a further favorable prognosis.
As with transplantation of other organs, surgery on the pancreas, in addition to rejection, is dangerous due to other side effects due to the disruption of varying degrees of secretory activity of the pancreas. In severe cases this leads to:
- to pancreatic diarrhea,
- to nausea and vomiting,
- to severe dehydration,
- to other dyspeptic phenomena,
- to general exhaustion.
After the procedure, the patient must continuously receive immunosuppressive drugs throughout his life to prevent rejection of foreign cells. The action of these drugs is aimed at reducing immune reactions - the production of antibodies. In turn, the lack of immunity increases the risk of developing any infection, even a simple one, which can become complicated and cause serious consequences.
Research continues on pancreatic transplantation from pigs—xenotransplantation. It is known that the anatomy of the gland and pork insulin are most similar to human insulin and differ from it in one amino acid. Before the discovery of insulin, an extract from the pig pancreas was used in the treatment of severe diabetes mellitus.
Why is a transplant performed?
Damaged pancreatic tissue is not restored. In cases of complicated diabetes mellitus, when the patient is on high doses of insulin, such surgical intervention saves the patient and gives a chance to restore the structure of beta cells. In a number of clinical studies, these cells were transplanted from donors into patients. As a result, the regulation of carbohydrate metabolism was restored. But at the same time, patients have to undergo powerful immunosuppressive therapy to prevent rejection of donor tissue.
Not all patients with type 1 diabetes are candidates for cell transplantation. There are strict indications:
- lack of results from the applied conservative treatment,
- insulin resistance,
- pronounced metabolic disorders in the body,
- severe complications of the disease.
Where is the operation performed and how much does it cost?
The procedure for replacing the islets of Langerhans is widely performed in the United States - this way diabetes of any type is treated in the early stages. One of the diabetes research institutes in Miami is doing this. It is not possible to completely cure diabetes in this way, but a good therapeutic effect is achieved, and the risks of severe complications are minimized.
The cost of such an intervention is about $100 thousand. Postoperative rehabilitation and immunosuppressive therapy range from $5 to $20 thousand. The cost of this treatment after surgery depends on the body's response to the transplanted cells.
Almost immediately after the manipulation, the pancreas begins to function normally on its own, and gradually its performance improves. The recovery process takes approximately 2 months.
Diabetes
The beta cells in the pancreas are complex. They belong to the endocrine part of the pancreas. If they are deprived of oxygen, it stops secreting insulin. After this, diabetes begins. This is a terrible and insidious disease that completely changes a person’s life.
Type I diabetes is an autoimmune disease. Here the beta compounds are attacked by the patient's immune system. In type II diabetes, tissue resistance to the action of insulin is observed. This is why blood sugar increases. This disease shortens the patient's life by 5-8 years.
The newest method of treatment is now the transformation of pancreatic duct cells into alpha compounds, followed by transformation into beta cells. In alpha cells, the Pax4 gene is activated here. This leads to the generation of new beta cells. This procedure can be carried out 3 times.
The research team is currently working on creating pharmacological molecules that will be able to cure diabetes patients in the future.
Damage to the endocrine pancreas
Islet cell dysfunction can occur for a variety of reasons. Often, the deficiency of these structures is related to congenital anomalies (genetic pathologies). Acquired damage to the islets of Langerhans develops as a result of viral and bacterial infections, chronic alcohol intoxication, and neurological diseases.
Insulin deficiency leads to type 1 diabetes. This disease occurs in childhood and young adulthood. Increased blood glucose leads to damage to blood vessels and nerves. With a deficiency of other islet cells, a hypoglycemic state develops and increased production of digestive juices. Increased production of hormones occurs with benign tumors of the tail of the pancreas.
Notes
- ↑ Langerhans P.
Beiträge zur mikroskopischen Anatomie der Bauchspeicheldrüse: Inaugural-Dissertation, zur Erlangung der Doctorwürde in der Medicine und Chirurgie vorgelegt der Medicinischen Facultät der Friedrich-Wilhelms-Universität zu Berlin und öffentlich zu vertheidigen am 18. Februar 1869. - Berlin : Buchdruckerei von Gustav Lange, 1869. - ↑ 1234567891011
Clinical diabetology / Efimov A. S., Skrobonskaya N. A. - 1st ed. - K.: Health, 1998. - 320 p. — 3000 copies. — ISBN 5-311-00917-9. - ↑ Zhukovsky M. A.
Pediatric endocrinology. — 3rd ed. - M.: Medicine, 1995. - 656 p. — 8000 copies. — ISBN 5-225-01167-5. - ↑ (English).
- ↑ (English). October 26, 2012.
- ↑ Proshchina A. E., Savelyev S. V.
// Bulletin of Experimental Biology and Medicine. — Ed. RAMS, 2013. - T. 155, No. 6. - P. 763-767.
Historical reference
Paul Langerhans, as a medical student working with Rudolf Virchow, in 1869 described clusters of cells in the pancreas that were different from the surrounding tissue, which were later named after him. In 1881, K.P. Ulezko-Stroganova first pointed out the endocrine role of these cells. The increatory function of the pancreas was proven in Strasbourg (Germany) in the clinic of the largest diabetologist Naunin Mering and Minkowski in 1889 - pancreatic diabetes was discovered and the role of the pancreas in its pathogenesis was proven for the first time. The Russian scientist L.V. Sobolev (1876-1919) in his dissertation “On the morphology of the pancreas when ligating its duct in diabetes and some other conditions” showed that ligation of the excretory duct of the pancreas leads the acinar (exocrine) section to complete atrophy, while pancreatic islets remain intact. Based on experiments, L.V. Sobolev came to the conclusion: “the function of the pancreatic islets is the regulation of carbohydrate metabolism in the body. The death of pancreatic islets and loss of this function causes a painful condition - diabetes mellitus."
Subsequently, thanks to a number of studies conducted by physiologists and pathophysiologists in various countries (pancreatectomy, selective necrosis of pancreatic beta cells with the chemical compound alloxan), new information was obtained about the increatory function of the pancreas.
In 1907, Lane & Bersley (University of Chicago) showed the difference between two types of islet cells, which they called type A (alpha cells) and type B (beta cells).
In 1909, the Belgian researcher Jan de Meyer proposed calling the secretion product of beta cells of the islets of Langerhans insulin (from the Latin insula - islet). However, direct evidence of the production of a hormone that affects carbohydrate metabolism could not be found.
In 1921, in the laboratory of physiology of Professor J. Macleod at the University of Toronto, the young Canadian surgeon Frederick Banting and his assistant medical student Charles Best managed to isolate insulin.
In 1955, Sanger and co-authors (Cambridge) managed to determine the amino acid sequence and structure of the insulin molecule.
In 1962, Marlin et al discovered that aqueous extracts of the pancreas were capable of increasing glycemia. The substance causing hyperglycemia was called “hyperglycemic-glycogenolytic factor.” It was glucagon, one of the main physiological antagonists of insulin.
In 1967, Donathan Steiner and co-authors (University of Chicago) managed to discover the insulin precursor protein, proinsulin. They showed that the synthesis of insulin by beta cells begins with the formation of a proinsulin molecule, from which C-peptide and an insulin molecule are subsequently split off as needed.
In 1973, John Ensik (University of Washington), as well as a number of American and European scientists, carried out work on the purification and synthesis of glucagon and somatostatin.
In 1976, Gudworth & Bottaggo discovered a genetic defect in the insulin molecule, revealing two types of the hormone: normal and abnormal. the latter is an antagonist to normal insulin.
In 1979, thanks to the research of Lacy & Kemp and co-authors, it became possible to transplant individual islets and beta cells, it was possible to separate the islets from the exocrine part of the pancreas and carry out transplantation in an experiment. In 1979-1980 When transplanting beta cells, a species-specific barrier is overcome (cells from healthy laboratory animals are implanted into sick animals of a different species).
In 1990, pancreatic islet cells were transplanted for the first time into a patient with diabetes mellitus.
Competition "bio/mol/text"-2019
This work was published in the category “Visually about the beloved” of the “bio/mol/text” competition-2019.
The general sponsor of the competition and partner of the Skoltech nomination is the Skoltech Center for Life Sciences.
Competition sponsor: the largest supplier of equipment, reagents and consumables for biological research and production.
The audience award was sponsored by BioVitrum.
"Book" sponsor of the competition - "Alpina Non-Fiction"
Let's meet the heroes of our article
Shark is a T cell.
Squid - B cell.
The anglerfish is an APC (antigen presenting cell) with HLA I and HLA II (human leukocyte antigen).
Chimera is a DE cell (dual-receptor-expressing cell, “bi-receptor” cell) with a TCR (T-cell receptor) and a BCR (B-cell receptor).
In order to understand what the unique “bi-receptor” lymphocyte does in type I diabetes (T1DM), let’s briefly talk about how the immune system works.
Before us is a formidable predator of body tissues - the antigen-presenting cell ( APC ). It absorbs uninvited guests—pathogens, such as bacteria—by phagocytosis.
After digestion, it presents part of the pathogen protein - antigen . In addition to HLA II, there is HLA I. Their meaning and functions are described in more detail in the article “Immunity: the fight against strangers and... our own” [1]. The APC swims into the lymph follicle and attracts naïve T cells, which circulate freely throughout the body.
Normally, the main task of a T-lymphocyte is to bind an antigen that is not found in the body, but enters it during pathological processes. The diversity of T lymphocytes is very large. It is due to the diversity of T-cell receptors, which is obtained due to recombinations of several genomic regions and the insertion of random nucleotides into the TCR gene [2]. Each of the cells is slightly different from the others due to the hypermutability of a short fragment of the N-terminal domain of the receptor and is capable of recognizing its potential antigen. This means that there is a possibility that the APC will encounter a T cell whose TCR will bind to the antigen presented on the HLA. However, this also means that the TCR can also approach the peptide that is found in the body's own normal cells. But no. Such T cells usually die in the thymus during a process called negative selection.
Once HLA II binds to the TCR, the desired T cells proliferate and release cytokines that help them differentiate. In order for these T cells to bind to HLA II, they have a protein called CD4 that “confirms” and stabilizes the binding.
After differentiation, CD4+ T cells emerge from the follicle and assume their commander role. They release cytokines that attract other cells (basophils, eosinophils, mast cells, etc.) to the site of infection and activate macrophages.
Meanwhile, naïve B cells (B lymphocytes) wait in the follicle for the antigen to arrive so they can take part in the fight against the pathogen.
They act through surface receptor molecules - B-cell receptors. B cell receptors are created through highly variable gene rearrangements. The naïve B cell waits for its BCR to bind to the antigen. It takes up and presents the antigen on its HLA II, and waits to meet a CD4+ T cell that has already been activated to the same antigen. The beauty of B-cell receptors (BCRs) is that random gene rearrangement produces such a wide variety of B-cell surface receptors that virtually any foreign antigen that enters the body is recognized by them.
The T-lymphocyte helps the B-lymphocyte to proliferate and begin to produce antibodies, which will be able to bind antigens in the same way as B-cell receptors. Antibodies bind to antigens on the surface of bacteria, and the other end (Fc-end) binds to receptors on macrophages ( starfish in the picture above), after which they phagocytose the bacterium. In addition, antibodies on the surface of bacteria activate the complement system and also prevent bacteria from attaching to body cells.
In 90% of patients with type 1 diabetes, there is a special variant of HLA II (HLA-DQ8): it binds better to an antigen that carries a relative negative charge at certain positions (positions 1 and 9). Insulin is considered an autoantigen in type 1 diabetes, that is, it is recognized as a foreign agent by the immune cells of patients.
Typically, any protein is not presented in its entirety: cells show only its most immunogenic part. In insulin, this part is amino acids 9–23 on the B chain (B:9–23). However, presentation of B:9–23 by either normal or mutant (HLA-DQ8) HLA II to CD4+ T cells from T1DM patients does not lead to their activation.
It was recently discovered that the blood of people with T1DM, in comparison with a healthy sample, is enriched in chimeras of T and B cells ( X cells ) [3]. The antibodies of these cells have a charge order of amino acid residues similar to insulin. The antibody sites have a better affinity for insulin-specific T cells and activate them, inciting the development of an autoimmune response to the insulin-producing beta cells of the islets of Langerhans.
Unfortunately, it is currently unknown what the nature of the occurrence of T- and B-cell chimeras is. The involvement of X cells in the development of other diseases also remains a mystery.
The authors suggest screening more at-risk subjects and using X-cell clonal enrichment data as a prognostic indicator. Better RNA-seq analysis is also needed to determine whether X cells are a distinct new cell type or present in the body as a subpopulation of one of the already known cell types.
The number of people in whom this discovery was made is small, but if the results are confirmed, it will be an exciting step towards better understanding the autoimmune nature of type 1 diabetes. It will be interesting to see future results from these researchers.
Links
| Diabetology |
|
| Non-immune forms of diabetes mellitus in children |
|
| Complications of treatment |
|
| Complications of diabetes | Acute (diabetic coma) Ketoacidosis Lactic acidosis Hyperosmolar coma Late Microangiopathy (Diabetic retinopathy, Diabetic nephropathy) Macroangiopathy Diabetic foot Diabetic neuropathy Mauriac syndrome Nobecourt syndrome Lesions of other organs and systems |